Tradeoffs between income, biodiversity, and ecosystem functioning during tropical rainforest conversion and agroforestry intensification
Abstract
Losses of biodiversity and ecosystem functioning due to rainforest destruction and agricultural intensification are prime concerns for science and society alike. Potentially, ecosystems show nonlinear responses to land-use intensification that would open management options with limited ecological losses but satisfying economic gains. However, multidisciplinary studies to quantify ecological losses and socioeconomic tradeoffs under different management options are rare. Here, we evaluate opposing land use strategies in cacao agroforestry in Sulawesi, Indonesia, by using data on species richness of nine plant and animal taxa, six related ecosystem functions, and on socioeconomic drivers of agroforestry expansion. Expansion of cacao cultivation by 230% in the last two decades was triggered not only by economic market mechanisms, but also by rarely considered cultural factors. Transformation from near-primary forest to agroforestry had little effect on overall species richness, but reduced plant biomass and carbon storage by ≈75% and species richness of forest-using species by ≈60%. In contrast, increased land use intensity in cacao agroforestry, coupled with a reduction in shade tree cover from 80% to 40%, caused only minor quantitative changes in biodiversity and maintained high levels of ecosystem functioning while doubling farmers' net income. However, unshaded systems further increased income by ≈40%, implying that current economic incentives and cultural preferences for new intensification practices put shaded systems at risk. We conclude that low-shade agroforestry provides the best available compromise between economic forces and ecological needs. Certification schemes for shade-grown crops may provide a market-based mechanism to slow down current intensification trends.
Keywords: agricultural economics, agroforestry management, land use change, plant–animal interactions, ecosystem goods and services
Global-scale conversion of tropical rainforests and agricultural intensification are major causes of biodiversity loss, and threaten ecosystem functioning, sustainable land use and local economies depending on natural resources (1–3). Developing strategies to reconcile human needs with the integrity of our environment is a major task for ecologists and socio-economists alike (4), but multitaxa studies are rare (5–6) and too little is known about the human dimension of land use changes (4, 7–11) and consequences for ecosystem functioning (1, 2, 12–14). Furthermore, most ecological and economic studies on ecosystem services are carried out separately so that information cannot be brought together (15). Particularly, quantitative data on potential tradeoffs between biodiversity loss and agricultural intensification including natural habitat conversion is missing. Two competing solutions propose either wildlife-friendly farming on the cost of agricultural yields or land sparing by agricultural intensification to minimize the demand for natural habitat (16). The evaluation of such opposing land use options depends on the concrete shape of the relationship between species richness and yields (16, 17). As biodiversity and ecosystem functions are likely to show nonlinear responses to increasing land use intensification, management alternatives with limited ecological losses and satisfying economic gains might exist (18).
Traditional agroforestry systems in the tropics resemble natural rainforests in many structural respects, and therefore have been suggested to be a promising wildlife-friendly land use strategy, conserving a significant proportion of tropical rainforest diversity while providing significant economic returns (17, 20).
Here, we use this habitat type to identify the cultural, economic, and geophysical causes of deforestation and agricultural intensification, and the ecological consequences for species richness and ecosystem functions. Our research was done at the margins of Lore Lindu National Park (LLNP) in Central Sulawesi, Indonesia, one of the core areas for the protection of the Wallacea biodiversity hotspot (21–23). Our focus was on agroforestry systems with cacao, which is the second most important tropical cash crop, cultivated on 6.99 million ha with a world production of 3.92 million metric tons and a production value of 4.93 billion € per year (FAO Statistical Databases: http://faostat.fao.org). Cacao cultivation takes place in a range of management systems from shaded agroforests to open monocultures (19). In our study region, we analyzed multistrata agroforestry systems with forest trees and planted trees in the shade canopy. For comparison, open cacao plantations without shade trees were included in socioeconomic studies. As biological indicator taxa, we used plants and insects because they represent ≈80% of all described species and determine important ecosystem processes (6, 12–14). To relate ecological changes to socioeconomic parameters, we used percent canopy cover as an indicator of forest tree loss and agroforestry intensification (17, 19–20). Furthermore, we quantified economic parameters of agricultural intensification and the often neglected cultural impacts on land use decisions.
Results
Land Cover Change.
Satellite image analyses show that, between 1972 and 2002, 15% (791 km2) of the study region was deforested and converted to agricultural land [supporting information (SI) Fig. 4]. Agroforestry areas, in which the cash crops coffee and cacao are grown under shade trees, expanded from 57.2 km2 in 1983 to 133.4 km2 in 2002. Compared with remaining forest, agroforestry sites are on average at lower elevations, on less steep slopes, closer to settlements and roads, and part of younger villages with more highly mechanized agriculture (SI Table 1).
Socioeconomic Drivers of Land Use Intensification.
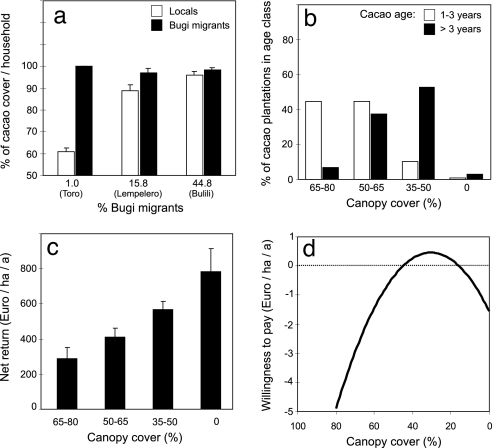
Expansion of cacao cultivation in our study region was triggered not only by favorable farmgate (producer) prices for cacao, but also by the introduction of intensified cacao farming techniques by migrants of Bugi ethnicity from southern Sulawesi to the study region in 1979. Our data from three representative villages with low, intermediate, and high proportions of migrants showed a parallel proportional increase of cacao cropping for autochtonous ethnic groups. Thus, cultural influences by migrant households changed the dominant livelihood strategy from a “food first” strategy based on irrigated rice to a “cash crop first” strategy (Fig. 1a), thereby increasing the pressure for forest conversion and intensification. The reduction of canopy cover for cacao agroforestry systems at harvest age (>3 years old, Fig. 1b) is one component of a more encompassing intensification syndrome, i.e., of a regularly observed coincidence of shade reduction with increased use of fertilizers and pesticides. This switch in livelihood strategies reflects the economic dominance of cacao agroforestry that, on average, provided two times higher mean annual net revenues compared with rice production (497 €/ha vs. 223 €/ha). Data from 199 cacao cropping households in 12 villages showed that intensified cacao production increases annual net returns from 285 €/ha on plots with 65–80% shade tree cover to 564 €/ha on plots with 35–50% cover, and to 780 €/ha in cacao plantations without shade trees (Fig. 1c). Parallel willingness-to-pay studies revealed economically motivated preferences for open cacao agroforestry with only ≈30% canopy cover (Fig. 1d). This figure is considerably less than the currently dominant agroforestry systems with 35–80% canopy cover (Fig. 1b). In sum, all socioeconomic analyses suggest a continuing trend toward intensified cacao agroforestry and shade tree removal.
Fig. 1.
Socioeconomic drivers of cacao agroforestry expansion and intensification. (a) Proportion of migrants and effects of ethnicity on land use decisions in three representative villages (GLM with percentage of cacao area per household (arcsine-square root transformed) as dependent variable: village: F = 3,74, P = 0.024, ethnicity: F = 10.56, P = 0.001, village × ethnicity: F = 3.82, P = 0.022, based on data from 636 households). (b) Relation of canopy cover in cacao agroforestry versus age of cacao trees. The lower shade levels of older agroforestry systems indicate a future trend of shade tree removal in young agroforestry systems. (c) Net returns of cacao agroforestry systems in relation to canopy cover for sites older than 3 years (log 10-transformed net returns: F = 6.94, P < 0.001, based on data from 199 households). (d) Average residual preferences for shading in local cacao agroforestry systems excluding other components of the intensification syndrome (fertilization, pesticide use) (pseudo-ρ2 = 35.6%; P < 0.0001; n = 249; nested logit analysis; ref. 37). Canopy cover refers to measurements of the shade trees above the cacao canopy.
Species Richness of Plants and Animals.
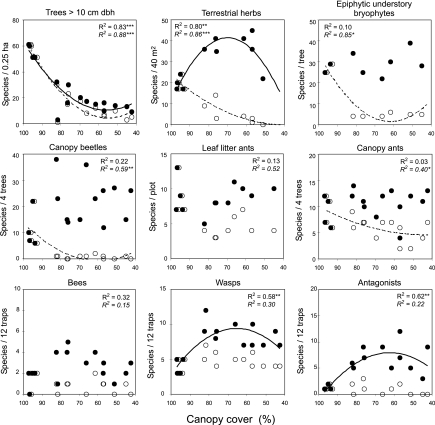
To understand the consequences of forest conversion and shade tree removal for biodiversity and ecosystem functioning, we selected four near-primary forest sites and 12 agroforestry plots covering a gradient in canopy cover reduction from ≈80% shading to ≈40%. At each site, we surveyed species richness of trees, herbs, epiphytic understory bryophytes, lower-canopy beetles, lower-canopy ants, leaf-litter ants, trap-nesting bees and wasps, and their antagonists. Additionally, we recorded densities of oribatid mites and collembolans in the soil. Surprisingly, total species richness of all studied species groups except trees was similar or even higher in agroforestry compared with near-primary forest sites. Species richness of herbs, bees, wasps, and their antagonists peaked at intermediate levels of canopy cover, whereas bryophytes, canopy beetles, and ants showed no significant correlation with canopy cover (Fig. 2). However, when we analyzed the distribution of the subset of species that also occurred in near-primary forest, we found that (i) only a small fraction of forest-based herbs, bryophytes, and beetles also colonized agroforestry systems; (ii) loss of forest-using ants was relatively low; and (iii) the few species of bees, wasps, and their antagonists found in the forest sites also occurred in agroforestry sites (SI Table 2). Overall, only ≈40% of the forest-based plant and insect species could be also observed in cacao agroforestry systems, with lower values for plant (6–43%) than for insect taxa (46–88%, SI Table 2).
Fig. 2.
Species richness of nine plant and insect groups along a gradient of canopy cover. Total species richness (filled circles, continuous lines, upper R2 values) and richness of species also recorded in near-primary forest (open circles, dashed lines, lower R2 values) are shown. The four plots with >90% canopy cover correspond to forest plots, the remainder correspond to cacao agroforestry systems. R2 values are based on polynomial regressions. Canopy cover refers to measurements of the shade trees above the cacao canopy.
Ecosystem Functioning.
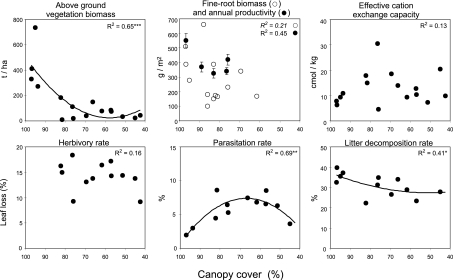
To evaluate the functional consequences of shifts in species richness and composition along the canopy cover gradient, we quantified key biotic interactions and ecosystem parameters. Standing above-ground plant biomass significantly decreased with reduced canopy cover, mainly due to the removal of large trees (Fig. 3). This reduction corresponded to a loss of ≈600 t CO2 per hectare of near-primary forest converted to cacao agroforestry systems, or a net release of ≈5.2 M t CO2 a−1 for the entire study region based on land-cover change statistics from satellite data (SI Fig. 4). Fine root biomass and annual fine root productivity also declined with lower canopy cover, implying additional loss of carbon from the below-ground vegetation component. Rates of herbivory on cacao trees did not change, whereas parasitism rates on bees and wasps peaked at intermediate shade cover and were related to the abundance of the respective functional group (P < 0.01). Removal of shade trees increased soil surface temperature by ≈4°C (linear regression y = 27.4 − 0.07 x, r2 = 0.43, n = 16, P = 0.005), and reduced relative air humidity at 2 m above ground by ≈12% (y = 73.5 + 0.21 x, r2 = 0.38, n = 16, P = 0.011). Accordingly, soil decomposition rates and abundance of soil arthropods (collembolans: r = 0.62, n = 12, P = 0.03; but not oribatid mites: r = 0.19, n = 12, P = 0.16) were lower in less shaded sites. Soil fertility parameters (pH, C-, N-, and P-contents, effective cation exchange capacity) did not change along the land-use gradient, conceivably due to counterbalancing effects of increasing fertilization or history of cultivation (Fig. 3).
Fig. 3.
Ecosystem functions along a gradient of canopy cover. R2 values are based on polynomial regressions. Cacao herbivory rates could not be measured in forest sites. The time-consuming quantification of fine root productivity was only done in a subset of sites. Canopy cover refers to measurements of the shade trees above the cacao canopy.
Discussion
Based on a description of the socioeconomic context of current land use changes, we analyzed tradeoffs between smallholder farm income, biodiversity, and ecosystem functioning along an agroforestry intensification gradient. Our study region covers typical stages of tropical land-use transition from natural forest across frontier clearings and small scale subsistence farming plots to intensive agricultural systems (18), and provides a case study with relevance for many tropical regions. Interestingly, land cover change was driven not only by relatively well known socioeconomic factors (8, 23, 24), but also by rarely considered, culturally mediated innovations: in our study region, immigrants of Bugi ethnicity from southern Sulawesi established intensified cacao farming practices and induced authochtonous groups to shift from a “food first” to a “cash crop first” strategy. This observation highlights the agricultural flexibility of local farmers, and indicates a potential pathway to establish sustainable management practices by extension service programs (4).
Based on the analysis of coffee agroforestry systems, it has recently been suggested that a win–win situation exists for canopy cover values that improve biodiversity preservation and economic performance (17), but ecological and economic studies on ecosystem services are rarely carried out together so that relevant information cannot be linked (15). The value of wildlife-friendly farming practices, such as shade-rich cacao agroforestry systems, compared with land sparing by agricultural intensification depends on the rarely analyzed shape of the relationship between biodiversity changes and yield increases (16, 18). In our study, we examine three axes of such land-use tradeoffs, namely net income, biodiversity, and selected ecosystem functions. Although we cover an unusually broad range of species groups and functions, it is possible that other taxa and functions might show other relationships to land-use change (5, 6).
Our results document that the conversion of rainforest to extensive cacao agroforestry with high shading levels strongly impacts plant biomass and carbon storage as well as diversity of forest-using plant and insect species. The transition from forest to cacao agroforestry resulted in a loss of ≈60% of the forest-based species with plant species being more strongly affected than mobile insect taxa. It seems reasonable to assume that rare, specialized, and endangered species are represented disproportionately high in this fraction (6), underlining the limitations of agroforestry for conservation of forest species (16). Unfortunately, detailed distributional and ecological data are lacking on most species encountered.
In comparison, additional changes in species richness and ecosystem functioning occurring along the investigated intensification gradient in cacao agroforestry were rather small. Only forest-based trees, herbs, and canopy ants tended to decline with shade cover reduction, whereas species richness of other groups, both forest- and non-forest-based species, remained constant. Other studies have reported a significant reduction in biodiversity along land-use intensification gradients in the tropics (5, 6, 19, 20, 25). This discrepancy is likely due to different extents in the analyzed land use gradients, with most studies also including agricultural systems completely devoid of trees. Our study did not cover cacao plantations without shade trees, which by definition are not agroforestry systems, but previous research in the same region for five groups of organisms (trees, herbs, birds, butterflies, and dung beetles) clearly indicates that completely unshaded systems harbor significantly lower species richness than shaded cacao systems (6). Similarly, other studies (5, 19, 20) document a final major loss of overall biodiversity at the transition from shaded agroforestry systems to intensively managed unshaded monocultures. Importantly, few studies have specifically considered shade cover gradients, which our study shows to include the most promising potential for an ecological and economic optimization of land use.
Ecosystem functioning also showed limited responses to shade cover reductions in cacao agroforestry systems for soil fertility, herbivory rates, plant biomass, and fine root productivity, indicating no further decline in carbon storage potential (26, 27). Only litter decomposition rates declined with more open conditions, presumably due to higher temperatures and lower humidity (28). The transition from shaded to open systems is likely to lead to abrupt state shifts in ecosystem functioning that may results in more regular pest outbreaks in cultivations, increased soil erosion, stronger and more common flood events, and in disruption of pollination as well as other ecosystem services (11, 15, 19, 24, 25). It has been shown that ecosystem functions such as pollination and biological pest control respond not only to local habitat management but also depend on distance from potential source populations in natural forest habitats (29–31). However, our study design specifically excluded landscape impacts by selecting all agroforestry study sites in proximity to the forest margin. Therefore, our results quantify local management effects but do not address the potential negative impact of isolation from source habitats on biodiversity.
In summary, our findings imply a concave, nonlinear relationship of canopy cover in agroforestry systems with biodiversity and ecosystem functioning. A doubling of income goes along with reduction of shade cover from >80% to 35–50%, most likely resulting only in limited losses of biodiversity and ecosystem functioning. In contrast, the conversion of forests to agroforestry systems in the first place as well as the complete removal of canopy trees as the final step of land use intensification, each result in disproportionate ecological losses.
Our analyses did not directly target effects of biodiversity on the ecological and economic resilience of agroecosystems against disturbance and resource degradation. However, biotic interactions appear surprisingly stable along the investigated intensification gradient. Furthermore, recent case studies demonstrate a positive relationship between functional group diversity and pollination or biological pest control in agroforestry systems (31, 32). In line with the insurance hypothesis, this finding suggests high overall ecosystem resilience facilitated by the high levels of species diversity in agroforestry systems (13, 29, 33–35).
To improve income and livelihoods at the rainforest margin, three basic land management options could be considered: further forest clearance, agroforestry intensification with complete shade tree removal, and thinning of high shade tree cover. We regard the latter option as the relatively most acceptable because the first two options will result in high, nearly certain, immediate losses of biodiversity and ecosystem functioning. Furthermore, if highest yield farming is used to minimize demand for farmland and spare remaining wilderness areas for conservation (16), this may have undesirable distributive effects. Observations in our study region indicate that only farming households already endowed with superior personal, social, and/or financial capital are likely to profit from this development.
Importantly, our results indicate that tradeoffs between income and overall biodiversity are less severe than tradeoffs between economic gains and losses of more specialized forest-based species. Therefore, socio-ecological models need to differentiate between approaches focusing on biodiversity-mediated ecosystem services and conservation of threatened taxa depending on natural habitats (36).
Although the clearance of little-disturbed rainforests could be slowed down administratively, e.g., by establishing national parks (SI Table 1), economic incentives are required to prevent agroforestry intensification beyond ecologically acceptable shade cover levels. Our data indicate a difference in net returns of ≈216 €/ha or 40% between low-shade cacao agroforestry and open plantations. Assuming average yields of 630 kg/ha, a price premium of 0.34 €/kg would be required for compensation. This estimate is similar to current price premiums for organic “fair-trade” cacao beans of at least 0.27 €/kg, suggesting that a market-based compensation for lower yields of shade-grown cacao could substantially slow down current intensification trends. Additionally, our willingness-to-pay (WTP) studies show a preference of farmers for low-shade agroforestry systems compared with open plantations, suggesting that even lower incentives could encourage the preservation of shaded agroforestry systems. The successfully established certification scheme for shade-grown coffee in Mesoamerica (17) shows that suitable market mechanisms are principally available that could also stabilize medium-intensive cacao agroforestry. Encouragement of cultural preferences for shaded cacao agroforestry systems and education of local farmers about unappreciated ecosystem services provided by shaded systems could further promote the implementation of certification schemes. Such market-based incentives will crucially determine whether shaded agroforestry systems remain important refugia for tropical biodiversity and sources of essential ecosystem services.
Materials and Methods
Study Region, Site-Characteristics, and Land-Use Change.
Ecological research took place around Toro village in the Kulawi valley at the western border of the Lore Lindu National Park in four forest and 12 agroforestry plots of 50 m × 50 m each. This region was chosen for its wide variety of cacao systems in close proximity to natural forest. It lacked unshaded cacao plantations. Mean distance of agroforestry sites to near-primary forest was 124 ± 18 m (SEM) and was not significantly correlated to canopy cover (r = −0.24, n = 12, P = 0.46). Canopy cover was measured by using a spherical densiometer, and temperature and relative humidity were measured with HOBO data loggers at eight points per site. Soil samples from the centre of each plot were extracted for lab analysis (pH, C-, N-, and P-contents, effective cation exchange capacity). Land-use change was quantified by using Landsat satellite images from 1972, 1983 (Landsat/MSS), and 2002 (Landsat/ETM+). Data were orthorectified and radiometrically preprocessed accounting for atmospheric and topographic distortions. A fuzzy logic classification system differentiated seven land cover categories: closed forests, open forests, agroforestry, annual crops, paddy fields, grass- and shrub-land, and water bodies.
Household and Village Surveys.
Comprehensive village censuses were conducted in Toro, Lempelero, and Bulili representing villages with contrasting socio-demographic dynamics (n = 636 households). On the regional scale, interviews with 301 households in 12 villages were conducted by using standardized, formal questionnaires on land-use and socio-demographic household characteristics (stratified random sample; 2004/2005). Choice experiments on economically motivated preferences for biodiversity, including shade trees in local cacao agroforestry systems, were administered to the same households (37). Further socioeconomic data (population density, age of the village, agricultural technologies) were collected during a village level survey in 2001.
Plant Surveys.
Trees ≥10 cm diameter at breast height (dbh) were sampled in plots of 50 m × 50 m subdivided into 25 subplots of 10 m × 10 m each, individually numbered, mapped, and measured to dbh, bole length, and total height. In each subplot, an area of 5 m × 5 m was sampled for trees of 2–9.9 cm dbh. Herbs were sampled in 10 plots of 2 m × 2 m randomly placed in each tree plot. Above-ground tree biomass was calculated by using the equation ln B = −3.375 + 0.948 × ln(D2 × H) (38) where B is aboveground biomass, D is dbh, and H is total tree height. Fine root standing mass was measured in 12 plots. Soil cores were taken at six locations per plot from the organic layer and the upper 50 cm of mineral soil. Fine root productivity was estimated with the sequential coring method conducted at 3-month intervals from February 2004 to February 2005. All tree root samples were cleaned and sorted under the stereo-microscope into live and dead particles. Epiphytic bryophytes were sampled on three understory trees per forest plot and three cacao trees per agroforestry plot, taking four samples of 200 cm2 each (one for each cardinal direction) on three different height levels of the trees (main stem up to 2 m or the first main branch, inner canopy, outer canopy).
Insect Surveys.
Lower canopy beetles and ants were sampled between 8.30–9.30 a.m. on four cacao trees per agroforestry site and four lower canopy trees per forest site, respectively, using knockdown fogging with a SwingFog TF 35 from December 2003 to January 2004. Bees, wasps, and their natural parasites were sampled with eight trap nests per site, of which four were placed at 2 m height and four at canopy height from September 2004 to October 2005. Trap nests consisted of bundles of reed internodes that provided nesting space for solitary bees and wasps and allowed quantification of trophic interactions (39). Litter-inhabiting ants were collected by using fish and honey baits, which were exposed on cacao leaves in eight agroforestry sites and natural tree leaves of similar size in the four forest sites. Eight baits were exposed in open and shaded patches of each site for 20 min twice per day (n = 32). Ants were collected from baits and bait-holding leaves and sorted into morphospecies, with all of them being identified to genus level.
Biotic Interactions.
Herbivory rates were quantified by randomly selecting 10 leaves ≈12 cm long per tree, from eight cacao trees on each of the 12 agroforestry plots. Using length and width, leaf surface was calculated and amount of leaf loss was quantified by counting numbers of eaten 0.25 cm2; averages of 80 leaves per site were taken. Rate of parasitism was calculated as the number of brood cells in trap nests attacked by natural enemies in relation to the total number of brood cells of bees and wasps.
Soil Arthropods and Litter Decomposition.
Soil microarthropods were sampled and extracted from soil (0–6 cm) and litter of four subplots within four forest and eight agroforestry plots at two sampling dates using 5-cm i.d. metal cores and a high gradient canister heat extractor. To assess litter decomposition rates, litter bags with cacao leaves were exposed in all forest and agroforest plots for eight months, and percent biomass loss was calculated from three litterbags per site and sampling date.
Statistical Analyses.
Most data were analyzed with SPSS version 11.5. Household data were analyzed by one-way ANOVA or GLM with first-order interactions. Data on species richness and ecosystem function were analyzed by simple and polynomial regression against canopy cover. We also tested for nonlinear relationships to take into account the assumption of maximum diversity at intermediate shading levels. If normality was not achieved, data were log-transformed (net returns) or arcsine-square root transformed (percentages). Means and standard errors (SEM) are given in the text and figures. We did not correct richness data for differences in the number of sampled individuals (e.g., by rarefaction or through estimators) because sampling was standardized and performed on a spatial grain size comparable to typical management units. Therefore, our data reflect the actual richness of the selected plots including effects of differing population densities rather than theoretical species numbers assuming constant densities on the different plots. For econometric analysis, (i) multinomial logit models were used to explore the relationships between land cover categories and geophysical, economic, and demographic factors; and (ii) nested logit models to determine economically motivated preferences for cacao shading intensity (NLOGIT 3.0). To minimize spatial autocorrelation effects spatially lagged slope, x,y coordinates and regular resampling of 5 × 5 pixels were applied to the models of SI Table 1.
Supplementary Material
Supporting Information
Acknowledgments
We thank Indonesian representatives in central and local government, Bogor Agricultural University (IPB), Tadulako University (UNTAD), and the villagers in the study region; the Indonesian and German project coordination; Jürgen Kluge and Pham Manh Cuong for technical support; M. van Noordwijk and two anonymous referees for comments on the current and K. Bawa, S. Cunningham, I. Perfecto, C. Perrings, and A. R. Smith for comments on an earlier version of the manuscript. This work was supported by Deutsche Forschungsgemeinschaft (DFG) Grant SFB 552-STORMA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Chapin FS, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, et al. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 2.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 3.Hoekstra JM, Boucher TM, Ricketts TH, Roberts C. Ecol Lett. 2005;8:23–29. [Google Scholar]
- 4.Luck GW, Ricketts TH, Daily GC, Imhoff M. Proc Natl Acad Sci USA. 2004;101:182–186. doi: 10.1073/pnas.2237148100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawton JH, Bignell DE, Bolton B, Bloemers GF, Eggleton P, Hammond PM, Hodda M, Holt RD, Larsen TB, Mawdsley NA, et al. Nature. 1998;391:72–76. [Google Scholar]
- 6.Schulze CH, Waltert M, Kessler PJA, Pitopang R, Shahabuddin, Veddeler D, Mühlenberg M, Gradstein SR, Leuschner C, Steffan-Dewenter I, Tscharntke T. Ecol Appl. 2004;14:1321–1333. [Google Scholar]
- 7.Balmford A, Bruner A, Cooper P, Costanza R, Farber S, Green RE, Jenkins M, Jefferiss P, Jessamy V, Madden J, et al. Science. 2002;297:950–953. doi: 10.1126/science.1073947. [DOI] [PubMed] [Google Scholar]
- 8.Bawa KS, Dayanandan S. Nature. 1997;386:562–563. [Google Scholar]
- 9.Liu J, Daily GC, Ehrlich PR, Luck GW. Nature. 2003;421:530–533. doi: 10.1038/nature01359. [DOI] [PubMed] [Google Scholar]
- 10.Adams WM, Aveling R, Brockington D, Dickson B, Elliott J, Hutton J, Roe D, Vira B, Wolmer W. Science. 2004;306:1146–1149. doi: 10.1126/science.1097920. [DOI] [PubMed] [Google Scholar]
- 11.Palmer M, Bernhardt, Chornesk E. Science. 2004;304:1251–1252. doi: 10.1126/science.1095780. [DOI] [PubMed] [Google Scholar]
- 12.Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, et al. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 13.Loreau M, Mouquet N, Gonzalez A. Proc Natl Acad Sci USA. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kremen C. Ecol Lett. 2005;8:468–479. doi: 10.1111/j.1461-0248.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter SR, DeFries R, Dietz T, Mooney HA, Polasky S, Reid WV, Scholes RJ. Science. 2006;314:257–258. doi: 10.1126/science.1131946. [DOI] [PubMed] [Google Scholar]
- 16.Green RE, Stephen JC, Scharlemann JPW, Balmford A. Science. 2005;307:550–555. doi: 10.1126/science.1106049. [DOI] [PubMed] [Google Scholar]
- 17.Perfecto I, Vandermeer J, Mas AA, Soto Pinto L. Ecol Econ. 2005;54:435–446. [Google Scholar]
- 18.DeFries RS, Foley JA, Asner GP. Front Ecol Environ. 2004;2:249–257. [Google Scholar]
- 19.Perfecto I, Rice RA, Greenberg R. BioScience. 1996;46:598–608. [Google Scholar]
- 20.Schroth G, da Fonseca GAB, Harvey CA, Gascon C, Vasconcelos HL, Izac A-MN. Agroforestry and Biodiversity Conservation in Tropical Landscapes. Washington, DC: Island; 2004. [Google Scholar]
- 21.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 22.Achard F, Eva HD, Stibig H-J, Mayaux P, Callego J, Richards T, Malingreau J-P. Science. 2002;297:999–1002. doi: 10.1126/science.1070656. [DOI] [PubMed] [Google Scholar]
- 23.Lepers E, Lambin EF, Janetos AC, DeFries R, Achard F, Ramankutty N, Scholes BioScience. 2005;55:115–124. [Google Scholar]
- 24.Maertens M, Zeller M, Birner R. Agric Econ. 2006;34:197–206. [Google Scholar]
- 25.Waltert M, Bobo S, Sainge NM, Fermon H, Mühlenberg M. Ecol App. 2005;15:1351–1366. [Google Scholar]
- 26.Guo LB, Gifford RM. Global Change Biol. 2002;8:345–360. [Google Scholar]
- 27.Nascimento HEM, Laurance WF. Ecol Appl. 2004;36(Suppl)(14):S127–S138. [Google Scholar]
- 28.Hairiah K, Sulistyani H, Suparyogo D, Purnomosidhi WP, Widodo RH, Van Noordwijk M. Forest Ecol Manag. 2006;224:45–57. [Google Scholar]
- 29.Ricketts TH, Daily GC, Ehrlich PR, Michener CD. Proc Natl Acad Sci USA. 2004;101:12579–12582. doi: 10.1073/pnas.0405147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanche KR, Ludwig JA, Cunningham SA. J Appl Ecol. 2006;43:1182–1187. [Google Scholar]
- 31.Klein A-M, Steffan-Dewenter I, Tscharntke T. J Anim Ecol. 2006;75:315–323. doi: 10.1111/j.1365-2656.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- 32.Klein A, Steffan-Dewenter I, Tscharntke T. Proc R Soc London Ser B. 2003;270:955–961. doi: 10.1098/rspb.2002.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmqvist T, Folke C, Nystrom M, Peterson G, Bengtsson J, Walker B, Norberg J. Front Ecol Environ. 2003;1:488–494. [Google Scholar]
- 34.Tscharntke T, Klein A-M, Kruess A, Steffan-Dewenter I, Thies C. Ecol Lett. 2005;8:857–874. [Google Scholar]
- 35.Naidoo R, Adamowicz WL. Proc Natl Acad Sci USA. 2005;102:16712–16716. doi: 10.1073/pnas.0508036102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tallis HM, Kareiva P. Trends Ecol Evol. 2006;21:562–568. doi: 10.1016/j.tree.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Hensher DA, Rose JM, Greene WH. Applied Choice Analysis: A Primer. Cambridge, UK: Cambridge Univ Press; 2005. [Google Scholar]
- 38.Brown S, Iverson LR. World Resource Rev. 1992;4:366–384. [Google Scholar]
- 39.Tscharntke T, Gathmann A, Steffan-Dewenter I. J Appl Ecol. 1998;35:708–719. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information