Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases - PubMed
Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases
Lyle Ralston et al. Plant Physiol. 2005 Apr.
Abstract
Flavonoids and isoflavonoids are major plant secondary metabolites that mediate diverse biological functions and exert significant ecological impacts. These compounds play important roles in many essential physiological processes. In addition, flavonoids and isoflavonoids have direct but complex effects on human health, ranging from reducing cholesterol levels and preventing certain cancers to improving women's health. In this study, we cloned and functionally characterized five soybean (Glycine max) chalcone isomerases (CHIs), key enzymes in the phenylpropanoid pathway that produces flavonoids and isoflavonoids. Gene expression and kinetics analysis suggest that the soybean type I CHI, which uses naringenin chalcone as substrate, is coordinately regulated with other flavonoid-specific genes, while the type II CHIs, which use a variety of chalcone substrates, are coordinately regulated with an isoflavonoid-specific gene and specifically activated by nodulation signals. Furthermore, we found that some of the newly identified soybean CHIs do not require the 4'-hydroxy moiety on the substrate for high enzyme activity. We then engineered yeast (Saccharomyces cerevisiae) to produce flavonoid and isoflavonoid compounds. When one of the type II CHIs was coexpressed with an isoflavone synthase, the enzyme catalyzing the first committed step of isoflavonoid biosynthesis, various chalcone substrates added to the culture media were converted to an assortment of isoflavanones and isoflavones. We also reconstructed the flavonoid pathway by coexpressing CHI with either flavanone 3beta-hydroxylase or flavone synthase II. The in vivo reconstruction of the flavonoid and isoflavonoid pathways in yeast provides a unique platform to study enzyme interactions and metabolic flux.
Figures
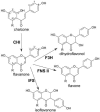
Partial flavonoid and isoflavonoid pathways. The numbering schemes of carbons for chalcone and flavanone are marked. The −R group is either H or OH. When R=H (OH), the common names are isoliquiritigenin (naringenin-chalcone), liquiritigenin (naringenin), DAI (genistein), dihydroxyflavone (apigenin), and kaempherol (quercetin) for chalcone, flavanone, isoflavone, flavone, and dihydroflavonol, respectively.

Amino acid sequence alignment of the alfalfa and soybean CHIs. Alfalfa MsaCHI1 and five soybean (GmaCHIs) are indicated. Light gray boxes surround similar residues. Bold residues surrounded by light gray boxes are conserved in greater than 50% of the sequences pictured. Bold white residues enclosed by dark gray boxes are absolutely conserved. Residues that line the active site are indicated by bold asterisks.
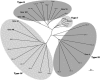
Phylogenetic comparison of deduced amino acid sequences of select CHI-like genes from higher plants and green algae. CHIs were grouped into families based on sequence homology and substrate specificity. The CHI sequences and corresponding GenBank accession numbers, TIGR Gene Index Numbers (TC), or PlantGDB EST Contig ID numbers (XXtuc) represented in this figure are as follows: Arabidopsis Ath2 (M86358), Ath3 (AY084729), Ath4 (AY063786); Chlamydomonas reinhardtii Chr3 (TC10476); soybean Gma1A (AY595413), Gma1B1 (AY595414), Gma1B2 (AY595419), Gma2 (AY595415), Gma3 (AY595416), Gma4 (AY595417); Gossypium arboreum Gar4 (GAtuc03-04-25.4968); Gossypium hirsutum Ghi4 (GHtuc03-04-25.551); Hordeum vulgare Hvu3 (HVtuc02-11-10.14749), Hvu4 (HVtuc02-11-10.15863); Lycopersicon esculentum Les3 (AY348871), Les4 (LEtuc02-10-21.8002); Lotus corniculatus Lco1A (AB073787), Lco1B (AB054801), Lco2 (AB054802); L. japonicus Lja1 (AJ548840); M. sativa Msa1 (M91079); M. truncatula Mtr1 (MTtuc03-04-26.11086); Oryza sativa Ora2 (AF474922), Ora4 (OStuc03-04-25.4740); Petunia hybrida Phy2 (AF233637); Phaseolus vulgaris Pvu1 (X16470); Pisum sativum Psa1 (U03433); Populus tremuloides Ptr3 (BU814723); Pueria lobata Plo1 (D63577); Solanum tuberosum Stu3 (STtuc02-10-23.12425). Soybean CHI sequences are bolded. Shaded ovals signify subfamily groupings.
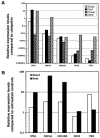
Quantitative RT-PCR analysis of the expression of soybean CHI gene family members compared with IFS and F3H in different tissues and in response to treatment with B. japonicum. A, The transcript levels of root, shoot, floral tissues, and immature embryos. B, The transcript levels after B. japonicum treatment compared to untreated controls. The relative transcript levels of IFS1, CHI1A, CHI1B2, CHI2, and F3H in the roots were 1,299.60%, 2.66%, 0.36%, 0.0018%, and 0.0002% compared to that of ubiquitin.
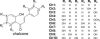
Schematic diagram of chalcones used in this study. Ch1, 2′-Hydroxychalcone; Ch2, 3,2′-dihydroxychalcone; Ch3, 4,2′-dihydroxychalcone; Ch4, 2′,4′-dihydroxychalcone; Ch5, 2′,4′-dihydroxy-4-methoxychalcone; Ch6, 2,2′,4′-trihydroxychalcone; Ch7, 2,2′,5′-trihydroxychalcone; Ch8, 4,2′,4′-trihydroxychalcone; Ch9, 4,2′, 5′-trihydroxychalcone; and Ch10, 4,2′,4′,6′-tetrahydroxychalcone.

HPLC profiles from yeast transformed with the empty vector (A and E), CHI1A (B and F), CHI1B2 (C and G), and CHI2 (D and H). Yeast cultures were fed naringenin chalcone (NC; sections A–D) or isoliquiritigenin (ISO; sections E–H). Some naringenin (NAR) was formed by spontaneous cyclization in yeast transformed by the empty vector (A). CHI1A cyclized both chalcones to their corresponding flavanones (B and F), as did CHI1B2 (C and G). CHI2 cyclized naringenin chalcone (D), and to a lesser degree isoliquiritigenin (H, compare to section A). Top sections (A–D) display A344, and bottom sections (E–H) display A312.
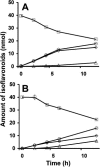
Time course of isoflavones and 2-hydroxyisoflavanones accumulation in yeast expressing IFS using naringenin (A) or liquiritigenin (B) as substrate. Each data point represents the average of three independent assays. Error bars represent
sd. The 2-hydroxyisoflavanones are represented by diamonds, isoflavones by triangles, combined IFS products by squares, and chalcones by squares.

HPLC profiles from yeast expressing the empty vector (A and F), CHI1A (B and G), coexpressing CHI1A and IFS (C and H), CHI1A and FNSII (D and I), or CHI1A and F3H (E and J). Yeast cultures were fed isoliquiritigenin (ISO; sections A–E) or naringenin chalcone (NC; sections F–J). Some naringenin (NAR) was formed by spontaneous cyclization in the yeast transformed by the empty vector (F). CHI1A cyclized both chalcones to the corresponding flavanone (B and G). IFS metabolized liquiritigenin (LIQ) to 2,7,4′-trihydroxyisoflavanone (3HI), 3,7,4′-trihydroxyflavanone (3HF), an unknown compounds (UN2), and DAI (C); and naringenin to 4HI and genistein (H). FNSII metabolized liquiritigenin to 7,4′-dihydroxyflavone (FLN; D); and naringenin to apigenin (API; I). F3H metabolized liquiritigenin to 3,7,4′-trihydroxyflavonol (FLA; E); and naringenin to dihydrokaempferol (DHK; J). All metabolite traces are displayed at 312 nm, because all metabolites except genistein are visible at this wavelength.
Similar articles
-
Dastmalchi M, Dhaubhadel S. Dastmalchi M, et al. Planta. 2015 Feb;241(2):507-23. doi: 10.1007/s00425-014-2200-5. Epub 2014 Nov 11. Planta. 2015. PMID: 25385351
-
Dastmalchi M, Bernards MA, Dhaubhadel S. Dastmalchi M, et al. Plant J. 2016 Mar;85(6):689-706. doi: 10.1111/tpj.13137. Plant J. 2016. PMID: 26856401
-
Shimada N, Aoki T, Sato S, Nakamura Y, Tabata S, Ayabe S. Shimada N, et al. Plant Physiol. 2003 Mar;131(3):941-51. doi: 10.1104/pp.004820. Plant Physiol. 2003. PMID: 12644647 Free PMC article.
-
Genetic and metabolic engineering of isoflavonoid biosynthesis.
Du H, Huang Y, Tang Y. Du H, et al. Appl Microbiol Biotechnol. 2010 May;86(5):1293-312. doi: 10.1007/s00253-010-2512-8. Epub 2010 Mar 23. Appl Microbiol Biotechnol. 2010. PMID: 20309543 Review.
-
Structure, function, and engineering of enzymes in isoflavonoid biosynthesis.
Wang X. Wang X. Funct Integr Genomics. 2011 Mar;11(1):13-22. doi: 10.1007/s10142-010-0197-9. Epub 2010 Oct 30. Funct Integr Genomics. 2011. PMID: 21052759 Review.
Cited by
-
Biochemical and Molecular Characterization of the Rice Chalcone Isomerase Family.
Park SI, Park HL, Bhoo SH, Lee SW, Cho MH. Park SI, et al. Plants (Basel). 2021 Sep 30;10(10):2064. doi: 10.3390/plants10102064. Plants (Basel). 2021. PMID: 34685873 Free PMC article.
-
Ban Z, Qin H, Mitchell AJ, Liu B, Zhang F, Weng JK, Dixon RA, Wang G. Ban Z, et al. Proc Natl Acad Sci U S A. 2018 May 29;115(22):E5223-E5232. doi: 10.1073/pnas.1802223115. Epub 2018 May 14. Proc Natl Acad Sci U S A. 2018. PMID: 29760092 Free PMC article.
-
Metabolic Engineering of Isoflavones: An Updated Overview.
Sohn SI, Pandian S, Oh YJ, Kang HJ, Cho WS, Cho YS. Sohn SI, et al. Front Plant Sci. 2021 Jun 7;12:670103. doi: 10.3389/fpls.2021.670103. eCollection 2021. Front Plant Sci. 2021. PMID: 34163508 Free PMC article. Review.
-
Engineered biosynthesis of natural products in heterologous hosts.
Luo Y, Li BZ, Liu D, Zhang L, Chen Y, Jia B, Zeng BX, Zhao H, Yuan YJ. Luo Y, et al. Chem Soc Rev. 2015 Aug 7;44(15):5265-90. doi: 10.1039/c5cs00025d. Epub 2015 May 11. Chem Soc Rev. 2015. PMID: 25960127 Free PMC article. Review.
-
Zhang J, Subramanian S, Zhang Y, Yu O. Zhang J, et al. Plant Physiol. 2007 Jun;144(2):741-51. doi: 10.1104/pp.106.095018. Epub 2007 Apr 13. Plant Physiol. 2007. PMID: 17434990 Free PMC article.
References
-
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S (2002) Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18: 298–305 - PubMed
-
- Bednar RA, Hadcock JR (1988) Purification and characterization of chalcone isomerase from soybeans. J Biol Chem 263: 9582–9588 - PubMed
-
- Beecher GR (2003) Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr 133: 3248S–3254S - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources