c-Src-mediated epithelial cell migration and invasion regulated by PDZ binding site - PubMed
c-Src-mediated epithelial cell migration and invasion regulated by PDZ binding site
Martin Baumgartner et al. Mol Cell Biol. 2008 Jan.
Abstract
c-Src tyrosine kinase controls proliferation, cell adhesion, and cell migration and is highly regulated. A novel regulatory mechanism to control c-Src function that has recently been identified involves the C-terminal amino acid sequence Gly-Glu-Asn-Leu (GENL) of c-Src as ligand for PDZ domains. Herein, we determined the biological relevance of this c-Src regulation in human breast epithelial cells. The intact GENL sequence maintained c-Src in an inactive state in starved cells and restricted c-Src functions that might lead to metastatic transformation under normal growth conditions. c-Src with a C-terminal Leu/Ala mutation in GENL (Src-A) promoted the activation and translocation of cortactin and focal adhesion kinase and increased the motility and persistence of cell migration on the basement membrane. Src-A promoted increased extracellular proteolytic activity, and in acinar cultures, it led to the escape of cells through the basement membrane into the surrounding matrix. We ascribe the regulatory function of C-terminal Leu to the role of GENL in modulating c-Src activity downstream of cell matrix adhesion. We propose that the C terminus of c-Src via its GENL sequence presents a mechanism that restricts c-Src in epithelia and prevents progression toward an invasive phenotype.
Figures
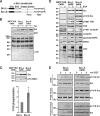
C-terminal Leu/Ala substitution in PDZ ligand sequence GENL increases Src activity in cells. (A) Domain structure of Src-L and the Src-A mutant with the C-terminal Leu/Ala substitution. (B) Wb of total cell lysates of the Src-L and Src-A cells grown in complete medium with or without Tet, using anti-Src2 and anti-pTyr antibodies. P40 is a nonspecific band appearing in MCF-10A cell lysates probed with anti-Src2 antibody and serves as a loading control. (C) Wb of total cell lysates of the MCF-10A, Src-L, and Src-A cells grown in complete medium using anti-Src and anti-pY416 Src antibodies. The bar diagram shows average pY416 Src values from three independent experiments (error bars are standard errors of the means, P = 0.034). (D) Src-L and Src-A cells were serum starved and then stimulated with EGF for 5 min. Wbs of total cell lysates are shown, using the antibodies indicated. (E) Wbs of cellular fractionation are shown of Src-L or Src-A cells stimulated for 1 or 5 min with EGF, using anti-Src2 and anti-pY416 Src antibodies. Fractions included in Wb are high-speed supernatant, cytosolic fraction (S100), P100 TX-100 sol., and P100 TX-100 insol.

Deregulated Src-A activity is independent of Csk and can be rescued by an alternative PDZ ligand. (A) HA-tagged full-length Src-L and Src-A or truncated (the kinase domain including the C terminus) SrcCT-L and SrcCT-A were expressed in reticulocyte lysates. Anti-HA immunoprecipitates were subjected to a kinase reaction with or without Csk. Tyr527 Src phosphorylation was detected by Wb. Expression controls for the individual proteins are shown below. (B) Wbs of the total cell lysates of Src-L and Src-A cells grown in complete medium using anti-pY416 Src, anti-pY527 Src, and anti-Src antibodies. (C) Src-L (GENL), Src-A (GENA), Src-STEV (STEV), and Src-STEA (STEA) were expressed in HEK293 cells. Wbs are shown of total cell lysates of cells grown in starvation medium using the antibodies indicated to the right of each panel. Cortactin was immunoprecipitated, and immunoprecipitates were subjected to anti-pTyr Wb.
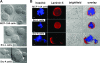
Src-A expression in MCF-10A cells disrupts acinar cultures. (A) Bright-field images of 11-day acinar cultures of MCF-10A, Src-L, and Src-A cells. Arrows indicate clusters of cells that protrude from the original acinar culture. (B) IF microscopy images, as in panel A, with anti-Ln-5 antibody (red). Nuclei stained with Hoechst compound are in blue. Bars correspond to 20 μm.
Src-A expression impairs cell polarization. (A) Wound-healing assay. Where indicated, dimethyl sulfoxide (DMSO) solvent or 10 μM of Src kinase inhibitor PP2 was added. Wound healing was monitored for 12 h by live video microscopy. Still pictures are shown from video microscopy at 0 and 12 h (see Movies S1 to S3 in the supplemental material). (B) The same still images shown in panel A comparing the MCF-10A, Src-L, and Src-A cells (see Movies S4 to S6 in the supplemental material). Cells at the leading edge at 0 h were tracked for 12 h and plotted. Each line represents the path of one single cell over time. Axes are in digital pixels (0.64 μm/dp). (C) The Golgi apparatus was visualized by using anti-giantin antibody, and the position of the Golgi body relative to that of the nucleus was determined. Approximately 100 cells from five different fields were analyzed for each cell line, and the percentage of cells with correctly oriented Golgi bodies was determined. Bars show percentages of correctly oriented Golgi bodies and corresponding standard errors of the means. (D) Ln-5 (red), the Golgi apparatus (green), and the nuclei (blue) were visualized in 11-day acinar cultures by confocal IF microscopy. Bars correspond to 20 μm.
Src-A expression impairs localization of E-cadherin. (A) Localization of E-cadherin during wound healing (8 h after wounding; top row) and in monolayers (middle row) or of β-catenin in monolayers (bottom row) was visualized by IF microscopy. For better visualization, IF staining is shown in the inverted mode. (B) Eleven-day acinar cultures of the MCF-10A, Src-L, and Src-A cells. Black and white images show inverted confocal IF of E-cadherin distribution. Color images show corresponding acinar cultures stained for E-cadherin (red), membrane protein palmitoylated 2 (green), and nuclei (blue). Arrows indicate the boundary between the acinar cultures and the ECM. Fourfold magnification of the framed area shows an escaping cell (arrowheads) with intact nucleus (n). (C) Confocal IF image of the Src-A acinar culture stained with anti-E-cadherin antibody. Arrowheads indicate punctuated E-cadherin staining in the escaping cell (fourfold magnification of the framed area). Bars correspond to 20 μm, unless otherwise stated.

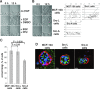
Src-A increases velocity and directional persistence of migration on BM. (A) Src-L or Src-A cells seeded onto BM of MCF-10A cells in the presence of dimethyl sulfoxide (DMSO) solvent or of 10 μM PP2. Images were recorded 1 h after seeding. Arrowheads indicate lamellipodia in solvent-treated cells. The area covered by individual cells was quantified using NIH image software. Bars show averages of areas covered and standard deviations (n = 19 cells from four randomly selected spots). (B and C) Src-L and Src-A cells were trypsinized and kept in suspension in starvation medium for 1 h at 37°C, seeded onto regular tissue culture plastic (B) or onto the BM of MCF-10A cells (C), and lysed at the indicated time points after seeding. Wb of total cell lysates, using anti-Src2 and anti-pY416 Src antibodies. (D) MCF-10A, Src-L, or Src-A cells were seeded as described in the legend to panel C. Cells were left unstimulated or treated with EGF. Cell movements were recorded by live cell video microscopy over 2 h, tracked, and plotted. (E) The average velocity and median directionality of cells treated as described in the legend to panel D were determined. Average velocities ± standard errors of the means (SEM) were as follows: MCF-10A cells, 0.96 ± 0.043 μm/min without EGF and 1.42 ± 0.1 with EGF; Src-L cells, 1.2 ± 0.10 μm/min without EGF and 1.64 ± 0.11 μm/min with EGF; Src-A cells, 1.45 ± 0.1 μm/min without EGF and 1.95 ± 0.24 μm/min with EGF. Medians ± SEM of directionality were as follows: MCF-10A cells, 0.31 ± 0.026 without EGF and 0.64 ± 0.067 with EGF; Src-L cells, 0.46 ± 0.081 without EGF and 0.55 ± 0.033 with EGF; Src-A cells, 0.67 ± 0.057 without EGF and 0.69 ± 0.046 with EGF (n = 45 randomly selected cells from four experiments; y-axis error bars are SEM).
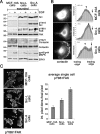
Src-A promotes phosphorylation of cortactin and FAK and leads to their accumulation toward the leading edge. (A) Wounds (160) were scratched into 50% confluent monolayers of starved MCF-10A, Src-L, and Src-A cells. Cells were then incubated without (−) or with (+) EGF for 12 h. Wb of total cell lysates, using the antibodies indicated, is shown. (B) MCF-10A, Src-L, or Src-A cells were seeded in starvation medium onto BM. Two hours after cells were seeded, cortactin was visualized by IF microscopy. Average fluorescence intensities of one-pixel-wide sections across clearly polarized cells (n = 6 polarized cells per cell line) were determined by using NIH image software and plotted. The orientation is trailing-to-leading edge; the dark gray bars are standard error of the mean (SEM) densities of grayscale fluorescence. Arrows indicate approximate positions of peak fluorescence near leading edges; arrowheads indicate the centers of gravity (peak fluorescence near the nucleus). (C) The MCF-10A, Src-L, and Src-A cells were seeded onto the BM as described in the legend to panel B. Phosphorylation of Y861 FAK was determined by confocal IF microscopy. Arrowheads indicate Y861 FAK phosphorylation at leading edges. The bar diagram shows the averages of integrated pixel densities per cell of 15 cells. Error bars are SEM.
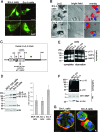
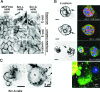
Increased Ln-5 processing in Src-A cells. (A) MT1-MMP (green) and nuclei (blue) were visualized by IF microscopy of nonpermeabilized Src-L or Src-A cells at 3 h after cells were seeded onto BM in starvation medium. (B) The MCF-10A, Src-L, and Src-A cells were seeded, and Ln-5 was visualized as described in the legend to panel A (Ln-5). Grayscale Ln-5 fluorescence was inverted and overlaid onto bright-field images (overlay). Areas with decreased Ln-5 are in red. (C) Schema of human Ln-5 γ2 chain with the putative MT1-MMP cleavage site within laminin-type EGF-like subdomains of domain III (according to rat Ln-5 γ2 chain [46]). (D) Wbs of the Ln-5 γ2 p155 and p105 chains in total MCF-10A, Src-L, and Src-A cell extracts using anti-Ln-5 antibody. Expression controls for Src and actin are shown. Pixel densities of the p155 and p105 bands from three independent experiments were quantified, and the ratio of p105/p155 was determined (error bars are standard errors of the means). (E) Gelatin zymography using supernatants of the MCF-10A, Src-L, and Src-A cells grown for 18 h in complete or starvation medium. (F) Gelatin zymography and Wb using membrane extracts of the MCF-10A, Src-L, and Src-A cells grown for 18 h in complete medium. SDS-PAGE was performed under nondenaturing conditions. (G) Ln-5 (red), MT1-MMP (green), and nuclei (blue) were visualized in 11-day acinar cultures by IF confocal microscopy. Bars correspond to 20 μm, unless otherwise stated.
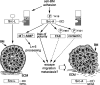
Model of the c-Src GENL sequence regulation of the invasion-promoting potential of c-Src. Replacement of the C-terminal leucine in the c-Src GENL sequence with alanine increased Src activity in cells and its potential to promote adhesion-induced cell migration by increasing the localized activation of cortactin and FAK and by promoting pericellular proteolysis. A combination thereof may lead to the establishment of impaired polarity during wound healing and, in acinar cultures, to the escape of cells into the surrounding ECM.
Similar articles
-
Avizienyte E, Fincham VJ, Brunton VG, Frame MC. Avizienyte E, et al. Mol Biol Cell. 2004 Jun;15(6):2794-803. doi: 10.1091/mbc.e03-12-0879. Epub 2004 Apr 9. Mol Biol Cell. 2004. PMID: 15075377 Free PMC article.
-
Franzen CA, Amargo E, Todorović V, Desai BV, Huda S, Mirzoeva S, Chiu K, Grzybowski BA, Chew TL, Green KJ, Pelling JC. Franzen CA, et al. Cancer Prev Res (Phila). 2009 Sep;2(9):830-41. doi: 10.1158/1940-6207.CAPR-09-0066. Epub 2009 Sep 8. Cancer Prev Res (Phila). 2009. PMID: 19737984
-
Chang LC, Huang CH, Cheng CH, Chen BH, Chen HC. Chang LC, et al. J Biomed Sci. 2005;12(4):571-85. doi: 10.1007/s11373-005-7212-5. Epub 2005 Nov 10. J Biomed Sci. 2005. PMID: 16132110
-
Biochemistry of the Src protein-tyrosine kinase: regulation by SH2 and SH3 domains.
Liu X, Pawson T. Liu X, et al. Recent Prog Horm Res. 1994;49:149-60. doi: 10.1016/b978-0-12-571149-4.50011-8. Recent Prog Horm Res. 1994. PMID: 7511826 Review.
-
Impact of interactions between normal and transformed epithelial cells and the relevance to cancer.
Hogan C. Hogan C. Cell Mol Life Sci. 2012 Jan;69(2):203-13. doi: 10.1007/s00018-011-0806-3. Epub 2011 Aug 30. Cell Mol Life Sci. 2012. PMID: 21877117 Free PMC article. Review.
Cited by
-
Rambur A, Lours-Calet C, Beaudoin C, Buñay J, Vialat M, Mirouse V, Trousson A, Renaud Y, Lobaccaro JA, Baron S, Morel L, de Joussineau C. Rambur A, et al. Nat Commun. 2020 May 8;11(1):2300. doi: 10.1038/s41467-020-16123-w. Nat Commun. 2020. PMID: 32385236 Free PMC article.
-
Raf kinase inhibitor RKIP inhibits MDA-9/syntenin-mediated metastasis in melanoma.
Das SK, Bhutia SK, Sokhi UK, Azab B, Su ZZ, Boukerche H, Anwar T, Moen EL, Chatterjee D, Pellecchia M, Sarkar D, Fisher PB. Das SK, et al. Cancer Res. 2012 Dec 1;72(23):6217-26. doi: 10.1158/0008-5472.CAN-12-0402. Epub 2012 Oct 11. Cancer Res. 2012. PMID: 23066033 Free PMC article.
-
p38gamma MAPK cooperates with c-Jun in trans-activating matrix metalloproteinase 9.
Loesch M, Zhi HY, Hou SW, Qi XM, Li RS, Basir Z, Iftner T, Cuenda A, Chen G. Loesch M, et al. J Biol Chem. 2010 May 14;285(20):15149-15158. doi: 10.1074/jbc.M110.105429. Epub 2010 Mar 15. J Biol Chem. 2010. PMID: 20231272 Free PMC article.
-
Integrin alpha9beta1: Unique signaling pathways reveal diverse biological roles.
Gupta SK, Vlahakis NE. Gupta SK, et al. Cell Adh Migr. 2010 Apr-Jun;4(2):194-8. doi: 10.4161/cam.4.2.10900. Epub 2010 Apr 8. Cell Adh Migr. 2010. PMID: 20179422 Free PMC article.
-
Yang M, Davis TB, Pflieger L, Nebozhyn MV, Loboda A, Wang H, Schell MJ, Thota R, Pledger WJ, Yeatman TJ. Yang M, et al. BMC Cancer. 2022 Mar 10;22(1):256. doi: 10.1186/s12885-022-09344-3. BMC Cancer. 2022. PMID: 35272617 Free PMC article.
References
-
- Artym, V. V., Y. Zhang, F. Seillier-Moiseiwitsch, K. M. Yamada, and S. C. Mueller. 2006. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 663034-3043. - PubMed
-
- Avizienyte, E., V. G. Brunton, V. J. Fincham, and M. C. Frame. 2005. The SRC-induced mesenchymal state in late-stage colon cancer cells. Cells Tissues Organs 17973-80. - PubMed
-
- Avizienyte, E., and M. C. Frame. 2005. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr. Opin. Cell Biol. 17542-547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous