Sustained activation of renal N-methyl-D-aspartate receptors decreases vitamin D synthesis: a possible role for glutamate on the onset of secondary HPT - PubMed
Sustained activation of renal N-methyl-D-aspartate receptors decreases vitamin D synthesis: a possible role for glutamate on the onset of secondary HPT
Eva Parisi et al. Am J Physiol Endocrinol Metab. 2010 Nov.
Abstract
N-methyl-D-aspartate (NMDA) receptors (NMDAR) are tetrameric amino acid receptors that act as membrane calcium channels. The presence of the receptor has been detected in the principal organs responsible for calcium homeostasis (kidney, bone, and parathyroid gland), pointing to a possible role in mineral metabolism. The aim of this study was to test the effect of NMDAR activation in the kidney and on 1,25(OH)₂D₃ synthesis. We determined the presence of NMDAR subunits in HK-2 (human kidney cells) cells and proved its functionality. NMDA treatment for 4 days induced a decrease in 1α-hydroxylase levels and 1,25(OH)₂D₃ synthesis through the activation of the MAPK/ERK pathway in HK-2 cells. In vivo administration of NMDA for 4 days also caused a decrease in blood 1,25(OH)₂D₃ levels in healthy animals and an increase in blood PTH levels. This increase in PTH induced a decrease in the urinary excretion of calcium and an increase in urinary excretion of phosphorous and sodium as well as in diuresis. Bone turnover markers also increased. Animals with 5/6 nephrectomy showed low levels of renal 1α-hydroxylase as well as high levels of renal glutamate compared with healthy animals. In conclusion, NMDAR activation in the kidney causes a decrease in 1,25(OH)₂D₃ synthesis, which induces an increase on PTH synthesis and release. In animals with chronic kidney disease, high renal levels of glutamate could be involved in the downregulation of 1α-hydroxylase expression.
Figures
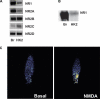
A: PCR for N-methyl-
d-aspartate (NMDA) receptor (NMDAR) subunits in human kidney 2 (HK-2) cells. Results show the presence of R1 and all R2 subunits. We used brain (Br) as a positive control. B: representative Western blot analysis showing NMDA R1 protein in HK-2 cells. C: fluo-4 determination of NMDAR activation. The entry of calcium caused by the activation of NMDAR can be visualized by the fluorochrome activation. Intracellular calcium levels are higher (measured as color intensity) after 1 min of NMDA treatment (500 μM).
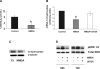
A: 1,25(OH)2D3 production by HK-2 cells after 4 days of NMDA treatment (500 μM/day). B: 1α-hydroxylase mRNA levels in HK-2 cells after 4 days of treatment with NMDA (500 μM/day; gray bars) with respect to the control ones (black bars). The inhibitory effect of NMDA treatment was abolished with the coincubation with the MAPK/ERK inhibitor U-0126 (white bar). Values are means ± SE. *P < 0.05 vs. control. C: representative Western blot for 1α-hydroxylase in HK-2 cells, control (Ct) or incubated with NMDA (500 μM). We used α-tubuline as load control. D: representative Western blot showing the activation of ERK1/2 on HK-2 cells after 60 and 72 h of NMDA (500 μM) incubation compared with the untreated cells or cells coincubated with U-0126 (10 μM). We used total ERK antibody as load control.
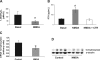
A: 1,25(OH)2D3 levels in normal animals before (black bars) and after NMDA treatment (10 mg·kg−1·day−1 for 4 days; gray bars). Values are means ± SE. *P < 0.05 vs. basal. B: PTH levels in normal rats before (black bars) and after NMDA treatment (10 mg·kg−1·day−1 for 4 days; gray bars) or NMDA plus calcitriol (NMDA + CTR; 10 ng/kg every other day). Values are means ± SE. *P < 0.05 vs basal. C: 1α-hydroxylase mRNA levels in kidney tissue from normal animals (control) and from animals treated for 4 days with NMDA (10 mg·kg−1·day−1). Values are means ± SE. *P < 0.05 vs. control. D: representative Western blot of kidney tissue showing a decrease in 1α-hydroxylase protein levels in 3 different animals treated with NMDA (10 mg·kg−1·day−1 for 4 days). We used α-tubulin as load control.
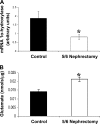
A: 1α-Hydroxylase protein levels in kidney tissue from healthy animals (black bars; control, n = 10) and from animals with 5/6 nephrectomy (white bars; n = 10). Values are means ± SE. *P < 0.05 vs. control. B: glutamate levels in kidney tissue from healthy rats (black bars) and from animals with 5/6 nephrectomy (white bars). Values are expressed as nmol of glutamate for μg of tissue protein. Values are means ± SE. *P < 0.05 vs. basal.
Similar articles
-
N-methyl-D-aspartate receptors are expressed in rat parathyroid gland and regulate PTH secretion.
Parisi E, Almadén Y, Ibarz M, Panizo S, Cardús A, Rodriguez M, Fernandez E, Valdivielso JM. Parisi E, et al. Am J Physiol Renal Physiol. 2009 Jun;296(6):F1291-6. doi: 10.1152/ajprenal.90557.2008. Epub 2009 Apr 8. Am J Physiol Renal Physiol. 2009. PMID: 19357180
-
Turner G, Coureau C, Rabin MR, Escoubet B, Hruby M, Walrant O, Silve C. Turner G, et al. Endocrinology. 1995 Sep;136(9):3751-8. doi: 10.1210/endo.136.9.7649081. Endocrinology. 1995. PMID: 7649081
-
Effects of oral phosphorus supplementation on mineral metabolism of renal transplant recipients.
Caravaca F, Fernández MA, Ruiz-Calero R, Cubero J, Aparicio A, Jimenez F, García MC. Caravaca F, et al. Nephrol Dial Transplant. 1998 Oct;13(10):2605-11. doi: 10.1093/ndt/13.10.2605. Nephrol Dial Transplant. 1998. PMID: 9794568
-
Cozzolino M, Gallieni M, Brancaccio D, Arcidiacono T, Bianchi G, Vezzoli G. Cozzolino M, et al. J Nephrol. 2006 Sep-Oct;19(5):566-77. J Nephrol. 2006. PMID: 17136683 Review.
Cited by
-
He K, Zhao Z, Hu X, Li Y. He K, et al. Appl Biochem Biotechnol. 2024 Aug;196(8):5354-5372. doi: 10.1007/s12010-023-04813-2. Epub 2023 Dec 29. Appl Biochem Biotechnol. 2024. PMID: 38157155
-
Glutamate-Gated NMDA Receptors: Insights into the Function and Signaling in the Kidney.
Valdivielso JM, Eritja À, Caus M, Bozic M. Valdivielso JM, et al. Biomolecules. 2020 Jul 15;10(7):1051. doi: 10.3390/biom10071051. Biomolecules. 2020. PMID: 32679780 Free PMC article. Review.
-
Husi H, Sanchez-Niño MD, Delles C, Mullen W, Vlahou A, Ortiz A, Mischak H. Husi H, et al. BMC Syst Biol. 2013 Oct 30;7:110. doi: 10.1186/1752-0509-7-110. BMC Syst Biol. 2013. PMID: 24172336 Free PMC article.
-
Functional NMDA receptors are expressed by human pulmonary artery smooth muscle cells.
Dong YN, Hsu FC, Koziol-White CJ, Stepanova V, Jude J, Gritsiuta A, Rue R, Mott R, Coulter DA, Panettieri RA Jr, Krymskaya VP, Takano H, Goncharova EA, Goncharov DA, Cines DB, Lynch DR. Dong YN, et al. Sci Rep. 2021 Apr 15;11(1):8205. doi: 10.1038/s41598-021-87667-0. Sci Rep. 2021. PMID: 33859248 Free PMC article.
-
Glutamate signaling in healthy and diseased bone.
Cowan RW, Seidlitz EP, Singh G. Cowan RW, et al. Front Endocrinol (Lausanne). 2012 Jul 19;3:89. doi: 10.3389/fendo.2012.00089. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22833735 Free PMC article.
References
-
- Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, Rodriguez M. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res 11: 970–976, 1996 - PubMed
-
- Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int 69: 33–43, 2006 - PubMed
-
- Beutner EH, Munson PL. Time course of urinary excretion of inorganic phosphate by rats after parathyroidectomy and after injection of parathyroid extract. Endocrinology 66: 610–616, 1960 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous