Troponin T nuclear localization and its role in aging skeletal muscle - PubMed
Troponin T nuclear localization and its role in aging skeletal muscle
Tan Zhang et al. Age (Dordr). 2013 Apr.
Abstract
Troponin T (TnT) is known to mediate the interaction between Tn complex and tropomyosin (Tm), which is essential for calcium-activated striated muscle contraction. This regulatory function takes place in the myoplasm, where TnT binds Tm. However, recent findings of troponin I and Tm nuclear translocation in Drosophila and mammalian cells imply other roles for the Tn-Tm complex. We hypothesized that TnT plays a nonclassical role through nuclear translocation. Immunoblotting with different antibodies targeting the NH2- or COOH-terminal region uncovered a pool of fast skeletal muscle TnT3 localized in the nuclear fraction of mouse skeletal muscle as either an intact or fragmented protein. Construction of TnT3-DsRed fusion proteins led to the further observation that TnT3 fragments are closely related to nucleolus and RNA polymerase activity, suggesting a role for TnT3 in regulating transcription. Functionally, overexpression of TnT3 fragments produced significant defects in nuclear shape and caused high levels of apoptosis. Interestingly, nuclear TnT3 and its fragments were highly regulated by aging, thus creating a possible link between the deleterious effects of TnT3 and sarcopenia. We propose that changes in nuclear TnT3 and its fragments cause the number of myonuclei to decrease with age, contributing to muscle damage and wasting.
Figures
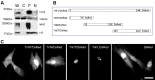
Troponin T3 is localized in the muscle nucleus. Whole cell lysis protein extract (W), cytosolic (C), myofibrillar (F), and nuclear (N) pools were prepared from the TA muscle. a A 35-kDa band showed that full-length TnT3 (TnFL) was most abundant in the myofibrillar pool and the whole cell lysate. A small portion was present in the nuclear pool. Histone H3 was used as a nuclear marker and detected in the nuclear pool and the whole cell lysis and the myofibrillar pool. Consistently, tubulin was mainly detected in the whole cell lysis and the cytosolic pool. b Schematic drawings of different TnT3 cDNA constructs with DsRed conjugation to the C-terminus. c Immunofluorescence images of various TnT3/DsRed proteins transiently expressed in the C2C12 cells. TnNT/DsRed, TnM/DsRed, and control DsRed are distributed throughout the cell. A distribution along the stress fibers was also noticed for TnM/DsRed. In contrast, TnCT/DsRed and TnFL/DsRed are mainly found in the nucleus and showed a punctate distribution pattern. Scale bar, 20 μm
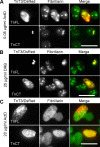
Transiently overexpressed TnFL/DsRed or TnCT/DsRed co-localized with fibrillarin and affected C2C12 nuclear morphology. TnT3/DsRed constructs were transiently expressed in C2C12 in growth medium for 2 days. Cells were then fixed, immunostained with fibrillarin antibody (nucleolar marker), Hoechst 33342, and examined by immunofluorescence microscopy. a Both TnFL/DsRed and TnCT/DsRed localized in the nucleolar area, yet TnFL/DsRed gathered fibrillarin around it and formed the nucleolar “cap” (arrows). TnCT/DsRed both co-localized with fibrillarin puncta and exhibited a diffuse nucleoplasmic distribution (arrow heads). In contrast, TnNT/DsRed showed no obvious effect on fibrillarin distribution (arrow heads). Data shown are representative of two independent experiments. b TnFL/DsRed and TnCT/DsRed were the only two constructs found in the nucleus, which changed from smooth and round or elliptic to irregular. In contrast, TnNT/DsRed showed the least effect on nuclear morphology, like control DsRed (arrows indicating nuclei from red fluorescence-positive cells). Scale bars, 25 μm. The graph quantifies the nuclei with irregular morphology for TnFL/DsRed-, TnCT/DsRed-, TnNT/DsRed-, and DsRed-transfected cells. The number of irregular nuclei is greater in TnFL/DsRed- and TnCT/DsRed- than TnNT/DsRed- and control DsRed-transfected cells (***P ≤ 0.001, n = 3; **P < 0.01, n = 3)
TnFL/DsRed and TnCT/DsRed showed different relationships with RNA polymerase I and II in C2C12 cells. C2C12 cells transiently expressing TnFL/DsRed, TnCT/DsRed, or TnNT/DsRed were immunostained with RNA polymerase I antibody (PRA194 [C-1], Pol I) or RNA polymerase II antibody (8WG16, Pol II), respectively. a Both TnFL/DsRed and TnCT/DsRed staining co-localized with Pol I in the nucleolar area (arrows). Unlike TnFL/DsRed, which showed mainly punctate nuclear distribution, TnCT/DsRed co-localized with Pol I in puncta and exhibited an additional diffused nucleoplasmic distribution. In contrast, TnNT/DsRed did not show any effect on RNA Pol I distribution. Data are representative of two independent experiments, and the numbers of cells analyzed are 94, 114, and 178 for TnFL/DsRed-, TnCT/DsRed-, and TnNT/DsRed-transfected cells, respectively. b TnFL/DsRed had a much stronger effect on Pol II enrichment in the nucleolar area (arrow heads). In contrast, most TnCT/DsRed or TnNT/DsRed showed no Pol II in the nucleolar area (arrows). Data are representative of two independent experiments, and the numbers of cells analyzed are 116, 110, and 175 for TnFL/DsRed-, TnCT/DsRed-, and TnNT/DsRed-transfected cells, respectively. Scale bars, 25 μm
Transcription inhibition with actinomycin D (ActD) or DRB in TnFL/DsRed or TnCT/DsRed transiently transfected C2C12 cells. a Incubating transfected cells in 0.05 μg/ml ActD for 3 h did not seem to affect fibrillarin distribution, yet TnFL/DsRed was dispersed into the nucleoplasm. In contrast, TnCT/DsRed remained punctate and co-localized with some fibrillarin puncta. Notably, the weak diffusive distribution of TnCT/DsRed in the nucleoplasm disappeared. b When only RNA Pol II was inhibited with 25 μg/ml DRB for 3 h, fibrillarin adopted a large punctate pattern in both TnFL/DsRed- and TnCT/DsRed-transfected cells. In contrast, TnCT/DsRed showed a diffuse nucleoplasmic distribution while TnFL/DsRed remained mainly punctate. c RNA Pol I and Pol II inhibition by 20 μg/ml ActD for 3 h resulted in diffuse fibrillarin dispersion into the nucleoplasm. Consistently, both TnFL/DsRed and TnCT/DsRed showed mainly a diffuse nuclear distribution pattern. Data are representative of two independent experiments, and at least 50 cells were analyzed per group. Scale bars, 25 μm
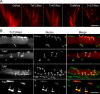
Subcellular localization of TnT3/DsRed proteins in mouse FDB fibers. TnT3/DsRed constructs and control plasmids were electroporated in vivo into the mouse FDB muscle. a Expression pattern of all constructs in whole isolated muscle. Control DsRed and TnNT/DsRed were expressed evenly throughout the muscle. In contrast, TnFL/DsRed, TnM/DsRed, and TnCT/DsRed showed a punctate distribution. b Higher magnification imaging analysis of individual myofibers showed that both TnFL/DsRed and TnCT/DsRed localized in some myonuclei. Additionally, TnFL/DsRed showed weak striated pattern. Notably, these two constructs localized at different subnuclear domains as revealed by their different co-localization pattern with Hoechst 33342 DNA staining. TnNT/DsRed also expressed in the myonuclei, but the myonuclear DNA staining pattern was not affected. TnM/DsRed mainly localized in the cytoplasm as small puncta, while most of it was highly ordered and reflects binding to myofibrils. Arrows point to nuclei in red fluorescence-positive areas. Scale bars, 800 μm (a), 50 μm (b). Fibers are representative of at least 200 fibers in each group from 2 to 13 experiments (DsRed, n = 3; TnFL/DsRed, n = 5; TnNT/DsRed, n = 6; TnM/DsRed, n = 2; TnCT/DsRed, n = 13)
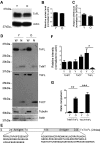
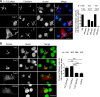
Western blot analysis of TnT3 and its fragments in young (3-month) and old (26–28-month) FVB mouse skeletal muscle. a TnT3 in TA whole muscle lysate, detected by a C-terminal-targeting antibody, shows decreased TnFL in old (O) compared to young (Y) mice. b TnFL expression was normalized to actin. c qPCR showing a slight decrease in TnT3, normalized to GADPH, mRNA in old (n = 5) compared to young (n = 6) mice. d Whole cell lysis (W) and nuclear protein fractions (N) from young and old mice were analyzed using TnT3 NT and CT antibodies. (e) The specific TnT3 N- and C-terminal regions targeted by the antibodies. f Normalized (against actin) TnNT, TnCT, and TnFL in the nuclear extracts were compared between young and old groups. TnCT tended to increase in the old nuclear extracts, and TnFL decreased dramatically (*P < 0.05, n = 3). TnNT did not change significantly. g The nuclear ratio between TnNT or TnCT and TnFL in both age groups was compared (**P = 0.012; ***P < 0.001, n = 3)
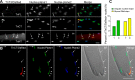
Transient expression of TnT3/DsRed proteins in mouse skeletal muscle in vivo affects myonuclear phenotype. a TnFL, TnCT, and TnNT nuclear expression in young FDB muscle fibers. Nuclei staining pattern was examined at two planes. Arrows indicate nuclei DNA staining in a clear, sharp punctate pattern; arrow heads indicate a blurred DNA staining pattern in the TnCT/DsRed-positive nucleus. b TnCT/DsRed expression in FDB muscle fibers from old FVB mice. Under the brightfield (BF) view, the TnCT/DsRed-positive nucleus showed abnormal shape and DNA stain (arrow heads) compared to neighboring normal untransfected nuclei (arrows). c Effects of TnCT/DsRed on nuclear shape and DNA staining pattern in young (Y, 271 nuclei) and old (O, 156 nuclei) muscle fibers from two experiments. Scale bars, 25 μm (a), 100 μm (b)
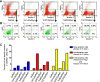
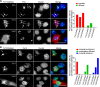
TnCT/DsRed and TnFL/DsRed overexpression induces apoptosis in C2C12 cells. Cells were analyzed with a FACSCalibur flow cytometer 48 h posttransfection. Data were collected on at least 100,000 freshly stained cells. Representative analyses of 7-AAD and Annexin V staining (b) followed pregating on DsRed (a). b Results were plotted as fluorescence intensity of Annexin V as a function of fluorescence intensity of 7-AAD. The numbers in each square represent the percentage of Annexin V−/7-AAD−(viable cells, left bottom corner); Annexin V+/7-AAD−(early apoptotic cells, right bottom corner); Annexin V+/7-AAD+(late apoptotic cells, right top corner); and Annexin V−/7-AAD+(broken cells, left top corner) in the DsRed-positive cell population. Data shown are representative of two independent experiments. c The percent of total apoptotic cells was obtained by adding early (Annexin V+/7-AAD−) and late apoptotic cells (Annexin V+/7-AAD+)
Similar articles
-
Zhang T, Pereyra AS, Wang ZM, Birbrair A, Reisz JA, Files DC, Purcell L, Feng X, Messi ML, Feng H, Chalovich J, Jin JP, Furdui C, Delbono O. Zhang T, et al. Aging Cell. 2016 Jun;15(3):488-98. doi: 10.1111/acel.12453. Epub 2016 Feb 19. Aging Cell. 2016. PMID: 26892246 Free PMC article.
-
Zhang T, Birbrair A, Delbono O. Zhang T, et al. Cytoskeleton (Hoboken). 2013 Mar;70(3):134-47. doi: 10.1002/cm.21095. Epub 2013 Feb 1. Cytoskeleton (Hoboken). 2013. PMID: 23378072 Free PMC article.
-
Troponin T3 associates with DNA consensus sequence that overlaps with p53 binding motifs.
Nunez Lopez YO, Messi ML, Pratley RE, Zhang T, Delbono O. Nunez Lopez YO, et al. Exp Gerontol. 2018 Jul 15;108:35-40. doi: 10.1016/j.exger.2018.03.012. Epub 2018 Mar 27. Exp Gerontol. 2018. PMID: 29596868 Free PMC article.
-
Jin JP. Jin JP. Int Rev Cell Mol Biol. 2016;321:1-28. doi: 10.1016/bs.ircmb.2015.09.002. Epub 2015 Nov 4. Int Rev Cell Mol Biol. 2016. PMID: 26811285 Review.
-
TNNT1, TNNT2, and TNNT3: Isoform genes, regulation, and structure-function relationships.
Wei B, Jin JP. Wei B, et al. Gene. 2016 May 10;582(1):1-13. doi: 10.1016/j.gene.2016.01.006. Epub 2016 Jan 13. Gene. 2016. PMID: 26774798 Free PMC article. Review.
Cited by
-
Skeletal muscle performance and ageing.
Tieland M, Trouwborst I, Clark BC. Tieland M, et al. J Cachexia Sarcopenia Muscle. 2018 Feb;9(1):3-19. doi: 10.1002/jcsm.12238. Epub 2017 Nov 19. J Cachexia Sarcopenia Muscle. 2018. PMID: 29151281 Free PMC article. Review.
-
Therapeutic exercise attenuates neutrophilic lung injury and skeletal muscle wasting.
Files DC, Liu C, Pereyra A, Wang ZM, Aggarwal NR, D'Alessio FR, Garibaldi BT, Mock JR, Singer BD, Feng X, Yammani RR, Zhang T, Lee AL, Philpott S, Lussier S, Purcell L, Chou J, Seeds M, King LS, Morris PE, Delbono O. Files DC, et al. Sci Transl Med. 2015 Mar 11;7(278):278ra32. doi: 10.1126/scitranslmed.3010283. Sci Transl Med. 2015. PMID: 25761888 Free PMC article. Clinical Trial.
-
Nuclear tropomyosin and troponin in striated muscle: new roles in a new locale?
Chase PB, Szczypinski MP, Soto EP. Chase PB, et al. J Muscle Res Cell Motil. 2013 Aug;34(3-4):275-84. doi: 10.1007/s10974-013-9356-7. Epub 2013 Aug 2. J Muscle Res Cell Motil. 2013. PMID: 23907338 Review.
-
Skeletal muscle neural progenitor cells exhibit properties of NG2-glia.
Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, Delbono O. Birbrair A, et al. Exp Cell Res. 2013 Jan 1;319(1):45-63. doi: 10.1016/j.yexcr.2012.09.008. Epub 2012 Sep 20. Exp Cell Res. 2013. PMID: 22999866 Free PMC article.
-
Chaves DF, Carvalho PC, Lima DB, Nicastro H, Lorenzeti FM, Siqueira-Filho M, Hirabara SM, Alves PH, Moresco JJ, Yates JR 3rd, Lancha AH Jr. Chaves DF, et al. J Proteome Res. 2013 Oct 4;12(10):4532-46. doi: 10.1021/pr400644x. Epub 2013 Sep 25. J Proteome Res. 2013. PMID: 24001182 Free PMC article.
References
-
- Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284(3):E481–E487. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical