Adrenergic regulation of HCN4 channel requires protein association with β2-adrenergic receptor - PubMed
- ️Sun Jan 01 2012
Adrenergic regulation of HCN4 channel requires protein association with β2-adrenergic receptor
Derek Greene et al. J Biol Chem. 2012.
Abstract
β(1)- and β(2)-adrenergic receptors utilize different signaling mechanisms to control cardiac function. Recent studies demonstrated that β(2)-adrenergic receptors (β(2)ARs) colocalize with some ion channels that are critical for proper cardiac function. Here, we demonstrate that β(2)ARs form protein complexes with the pacemaker HCN4 channel, as well as with other subtypes of HCN channels. The adrenergic receptor-binding site was identified at a proximal region of the N-terminal tail of the HCN4 channel. A synthetic peptide derived from the β(2)AR-binding domain of the HCN4 channel disrupted interaction between HCN4 and β(2)AR. In addition, treatment with this peptide prevented adrenergic augmentation of pacemaker currents and spontaneous contraction rates but did not affect adrenergic regulation of voltage-gated calcium currents. These results suggest that the ion channel-receptor complex is a critical mechanism in ion channel regulation.
Figures

Multiple HCN channel subtypes selectively associate with β2AR but not with other ARs. A, protein complex formation between the HCN4 channel and β2AR. The indicated combinations of V5 epitope-tagged HCN4 and FLAG-tagged ARs were transiently expressed in HEK cells and subjected to immunoprecipitation (IP) using anti-FLAG antibody. Upper panel, β2AR selectively coprecipitated the HCN4 channel. Middle panel, immunoprecipitated FLAG-tagged ARs. Lower panel, summary of the quantification of three independent experiments. B, upper panel, β2AR coprecipitated HCN1-V5. Lower panel, summary of the quantification of three independent experiments. C, upper panel, β2AR coprecipitated HCN2-GFP. Lower panel, summary of the quantification of three independent experiments. Error bars show S.E.

Mapping analyses of the β2AR-binding site of the HCN4 channel. A, schematic diagram of the HCN4 channel showing the membrane topology and key residue numbers. B, immunoprecipitation (IP) using FLAG-β2AR and the GFP-tagged full-length HCN4 channel (amino acids (aa) 1–1203) and fragments containing the N terminus (amino acids 1–260) and the C terminus (amino acids 521–1203). C, immunoprecipitation using FLAG-β2AR and GFP-tagged N-terminal fragments. GFP-HCN4(225–260) was the shortest fragment demonstrating the interaction. D, summary of mapping analyses. Error bars show S.E. E, alignment of the β2AR-binding site for the HCN channel family. Conserved amino acids are boxed. The computer-predicted secondary structure for the helix (H) and the extended helix (E) are indicated. h, human.
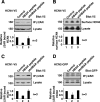
Synthetic peptide HBAR disrupts the HCN channel-β2AR interaction. A, pretreatment with HBAR but not shHBAR reduced co-immunoprecipitation of the HCN4 channel. HCN4-V5 and FLAG-β2AR were coexpressed in HEK cells and subjected to immunoprecipitation (IP) using anti-FLAG antibody. B, pretreatment with HBAR did not alter HCN4 protein amount at the plasma membrane (surface HCN4) and in the whole cell lysate. C, pretreatment with HBAR reduced β2AR-bound HCN1. D, pretreatment with HBAR reduced β2AR-bound HCN2. Error bars show S.E.

Adrenergic regulation of If in rat SAN cells. A, current traces of If in the same cell showing before (black) and after (red) application of ISO. The cell was clamped at a holding potential of −50 mV, and 1-s step hyperpolarizations between −50 and −150 mV with 10-mV steps were applied. B, current traces from a voltage step to −110 mV shown in A depicting augmentation of If by ISO. C, summary of pooled data for voltage-current relationships of If and adrenergic modulation using the voltage protocol used in A. Slow tail currents of If at −50 mV evoked after hyperpolarization were measured and normalized to the tail current evoked by a step to −150 mV under the control conditions (○). After applying ISO, If was reevaluated and compared with the control (●). D, the voltage ramp protocol (upper) evoked a hyperpolarization-activated current, which was inhibited by ZD7288 (lower). E, current traces evoked by the voltage ramp protocol showing ISO-mediated If changes (lower). Upper, the points where potentials are at −110 and −70 mV. Differences in the induced currents between I(−110 mV) and I(−70 mV) are designated I(Δ−40 mV) and were used to evaluate If amplitude. F, summary of pooled data showing rapid augmentation of If after ISO application presented as normalized I(Δ−40 mV). Error bars show S.E.

HBAR interferes with adrenergic augmentation of If by voltage steps. A, current traces evoked by step hyperpolarization showing before (left) and after (middle) application of ISO from HBAR-treated cells. Also shown are current traces from a voltage step to −110 mV demonstrating that ISO application did not induce any change in If (right). B, current traces showing that treatment with the control shHBAR peptide maintained adrenergic augmentation of If. C, current traces evoked by voltage ramp from HBAR-treated cells showing the null response to ISO application. D, current traces evoked by voltage ramp from shHBAR-treated cells showing maintained ISO-mediated augmentation. E, pooled data of voltage ramp experiments. HBAR treatment blunted ISO responses. The black box indicates the presence of ISO. F, histogram of normalized I(Δ−40 mV) at t = 60 s from E and Fig. 4F (SAN cells) at the indicated conditions. *, < 0.05. Error bars show S.E.
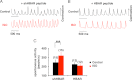
HBAR eliminates adrenergic facilitation of spontaneous beating of SAN cells. A, spontaneous contraction of SAN cells was monitored by live cell imaging. Optical signals from the edges of cells were measured to detect cell movements. Spontaneous beating of shHBAR-treated cells was accelerated by ISO. B, HBAR-treated SAN cells did not respond to ISO. C, summary of the adrenergic effect on spontaneous beating. **, < 0.01. Error bars show S.E.
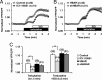
PKA activation is not disturbed by HBAR treatment. A, PKA activity was measured using AKAR. Application of a β2AR-selective agonist, terbutaline, was followed by application of a nonselective agonist for β1AR + β2AR, ISO. YFP/CFP ratios calculated from fluorescence intensities measured at the cytoplasm are plotted against time. Ratios were normalized to that at t = 0. Application of 5 n
mICI-118551 suppressed most of the terbutaline-induced PKA activity. B, PKA activity in HBAR- or shHBAR-treated cells. C, summary of PKA activity shown in A and B. ICI-118551 significantly suppressed AKAR responses induced by both terbutaline and ISO, but neither HBAR nor shHBAR treatment altered AR-mediated PKA activation. **, < 0.01. Error bars show S.E.

Voltage-gated calcium currents from ventricular cardiomyocyte are not affected by HBAR peptides. A, current traces of voltage-gated calcium currents before (upper left) and after (upper right) application of ISO. Cells were clamped at a holding potential of −80 mV and depolarized to potentials between −80 and 50 mV in steps of 10 mV for 500 ms. Lower, voltage-current relationship obtained from the traces shown. B, time course for adrenergic modulation of calcium currents from untreated (open circles), HBAR-treated (blue circles), and shHBAR-treated (red triangles) myocytes are shown. Inset, current traces at t = 0 and t = 1 min evoked by 100-ms step depolarizations to −10 mV from a holding potential of −80 mV. C, summary of adrenergic modulation of calcium current. A histogram of currents at t = 1 min in B is shown for comparison. Error bars show S.E.
Similar articles
-
Liao Z, Lockhead D, Larson ED, Proenza C. Liao Z, et al. J Gen Physiol. 2010 Sep;136(3):247-58. doi: 10.1085/jgp.201010488. Epub 2010 Aug 16. J Gen Physiol. 2010. PMID: 20713547 Free PMC article.
-
Li CH, Zhang Q, Teng B, Mustafa SJ, Huang JY, Yu HG. Li CH, et al. Am J Physiol Cell Physiol. 2008 Jan;294(1):C355-62. doi: 10.1152/ajpcell.00236.2007. Epub 2007 Oct 31. Am J Physiol Cell Physiol. 2008. PMID: 17977941 Free PMC article.
-
Ye B, Nerbonne JM. Ye B, et al. J Biol Chem. 2009 Sep 18;284(38):25553-9. doi: 10.1074/jbc.M109.007583. Epub 2009 Jul 1. J Biol Chem. 2009. PMID: 19574228 Free PMC article.
-
Baruscotti M, Bottelli G, Milanesi R, DiFrancesco JC, DiFrancesco D. Baruscotti M, et al. Pflugers Arch. 2010 Jul;460(2):405-15. doi: 10.1007/s00424-010-0810-8. Epub 2010 Mar 8. Pflugers Arch. 2010. PMID: 20213494 Review.
-
The funny current: cellular basis for the control of heart rate.
DiFrancesco D, Borer JS. DiFrancesco D, et al. Drugs. 2007;67 Suppl 2:15-24. doi: 10.2165/00003495-200767002-00003. Drugs. 2007. PMID: 17999560 Review.
Cited by
-
Joyce W, Pan YK, Garvey K, Saxena V, Perry SF. Joyce W, et al. Acta Physiol (Oxf). 2022 Aug;235(4):e13849. doi: 10.1111/apha.13849. Epub 2022 Jun 16. Acta Physiol (Oxf). 2022. PMID: 35665450 Free PMC article.
-
Bai Q, Zhang Y, Ban Y, Liu H, Yao X. Bai Q, et al. PLoS One. 2013 Jul 29;8(7):e68138. doi: 10.1371/journal.pone.0068138. Print 2013. PLoS One. 2013. PMID: 23922653 Free PMC article.
-
Compartmentalized cAMP signalling and control of cardiac rhythm.
Tomek J, Zaccolo M. Tomek J, et al. Philos Trans R Soc Lond B Biol Sci. 2023 Jun 19;378(1879):20220172. doi: 10.1098/rstb.2022.0172. Epub 2023 May 1. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37122225 Free PMC article. Review.
-
Genetic Complexity of Sinoatrial Node Dysfunction.
Wallace MJ, El Refaey M, Mesirca P, Hund TJ, Mangoni ME, Mohler PJ. Wallace MJ, et al. Front Genet. 2021 Apr 1;12:654925. doi: 10.3389/fgene.2021.654925. eCollection 2021. Front Genet. 2021. PMID: 33868385 Free PMC article. Review.
-
Naumova N, Iop L. Naumova N, et al. Front Bioeng Biotechnol. 2021 Aug 2;9:673477. doi: 10.3389/fbioe.2021.673477. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34409019 Free PMC article. Review.
References
-
- Mika D., Leroy J., Vandecasteele G., Fischmeister R. (2012) PDEs create local domains of cAMP signaling. J. Mol. Cell. Cardiol. 52, 323–329 - PubMed
-
- Zaccolo M., Pozzan T. (2002) Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science 295, 1711–1715 - PubMed
-
- Nikolaev V. O., Moshkov A., Lyon A. R., Miragoli M., Novak P., Paur H., Lohse M. J., Korchev Y. E., Harding S. E., Gorelik J. (2010) β2-Adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327, 1653–1657 - PubMed
-
- Conti M., Beavo J. (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 76, 481–511 - PubMed
-
- Davare M. A., Avdonin V., Hall D. D., Peden E. M., Burette A., Weinberg R. J., Horne M. C., Hoshi T., Hell J. W. (2001) A β2-adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293, 98–101 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials