Drug-evoked synaptic plasticity causing addictive behavior - PubMed
- ️Tue Jan 01 2013
Review
Drug-evoked synaptic plasticity causing addictive behavior
Christian Lüscher. J Neurosci. 2013.
No abstract available
Figures
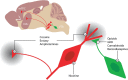
The mesocorticolimbic dopamine system as an initial target of addictive drugs. The VTA, at the origin of the mesocorticolimbic system, is composed of dopamine projection neurons that are under inhibitory control of GABA interneurons (somtimes described as an independent nucleus at the tail of the VTA, the rostromedial tegmentum). The main targets are the NAc and the mPFC. Addictive drugs cause an increase in mesocorticolimbic dopamine through three distinct cellular mechanisms: direct activation of dopamine neurons (e.g., nicotine); indirect disinhibition of dopamine neurons [opioids, gamma-hydroxybutyric acid (GHB), cannabinoids, and benzodiazepines]; as well as interference with dopamine reuptake (cocaine, ecstasy, and amphetamines). Note that the latter group also increases dopamine in the VTA itself, owing to the perturbation of the reuptake of dendritically released dopamine (modified from Lüscher and Malenka, 2011, with permission).
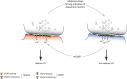
Drug-evoked synaptic plasticity in dopamine neurons of the ventral tegmental area. Addictive drugs or strong stimulation of dopamine neurons causes a synaptic plasticity on the excitatory afferents. This plasticity is expressed by the dual exchange of AMPA and NMDA receptors: GluA2-lacking AMPA receptors and GluN3A-containing NMDA receptors are inserted. As a consequence calcium enters the postsynaptic cell more readily when the membrane is hyperpolarized, which inverts the rules for activity-dependent synaptic plasticity. At baseline, LTP is induced when glutamate is released onto depolarized dopamine neurons (Hebbian LTP that is NMDAR dependent), while after cocaine exposure LTP can be observed when glutamate transmission coincides with a hyperpolarization (anti-Hebbian LTP that is AMPAR dependent). Normal transmission is restored by the activation of mGluR1. Together, addictive drugs evoked a permissive metaplasticity at excitatory afferents onto VTA dopamine neurons, which gate subsequent adaptations downstream (see text).
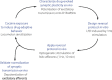
Experimental blueprint to establish causal relationship between drug-evoked synaptic plasticity and drug-adaptive behavior. In gray example experiments are from a proof-of-principle study (Pascoli et al., 2011). For further explanation see text.
Similar articles
-
The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens.
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. Russo SJ, et al. Trends Neurosci. 2010 Jun;33(6):267-76. doi: 10.1016/j.tins.2010.02.002. Epub 2010 Mar 5. Trends Neurosci. 2010. PMID: 20207024 Free PMC article. Review.
-
Synaptic plasticity during the cholinergic theta-frequency oscillation in vitro.
Huerta PT, Lisman JE. Huerta PT, et al. Hippocampus. 1996;6(1):58-61. doi: 10.1002/(SICI)1098-1063(1996)6:1<58::AID-HIPO10>3.0.CO;2-J. Hippocampus. 1996. PMID: 8878743 No abstract available.
-
Short-term synaptic plasticity: a comparison of two synapses.
Blitz DM, Foster KA, Regehr WG. Blitz DM, et al. Nat Rev Neurosci. 2004 Aug;5(8):630-40. doi: 10.1038/nrn1475. Nat Rev Neurosci. 2004. PMID: 15263893 Review. No abstract available.
-
The diversity of synaptic plasticity.
Chapman PF. Chapman PF. Nat Neurosci. 2001 Jun;4(6):556-8. doi: 10.1038/88367. Nat Neurosci. 2001. PMID: 11369931 No abstract available.
-
Sun W. Sun W. Curr Drug Abuse Rev. 2011 Dec;4(4):270-85. doi: 10.2174/1874473711104040270. Curr Drug Abuse Rev. 2011. PMID: 21834753 Review.
Cited by
-
Brain Stimulation in Addiction.
Salling MC, Martinez D. Salling MC, et al. Neuropsychopharmacology. 2016 Nov;41(12):2798-2809. doi: 10.1038/npp.2016.80. Epub 2016 May 31. Neuropsychopharmacology. 2016. PMID: 27240657 Free PMC article. Review.
-
The Synaptic Theory of Memory: A Historical Survey and Reconciliation of Recent Opposition.
Langille JJ, Brown RE. Langille JJ, et al. Front Syst Neurosci. 2018 Oct 26;12:52. doi: 10.3389/fnsys.2018.00052. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30416432 Free PMC article.
-
Bailey CP, Husbands SM. Bailey CP, et al. Expert Opin Drug Discov. 2014 Nov;9(11):1333-44. doi: 10.1517/17460441.2014.964203. Epub 2014 Sep 25. Expert Opin Drug Discov. 2014. PMID: 25253272 Free PMC article. Review.
-
Multiple faces of BDNF in cocaine addiction.
Li X, Wolf ME. Li X, et al. Behav Brain Res. 2015 Feb 15;279:240-54. doi: 10.1016/j.bbr.2014.11.018. Epub 2014 Nov 15. Behav Brain Res. 2015. PMID: 25449839 Free PMC article. Review.
-
Peeters LD, Wills LJ, Cuozzo AM, Ivanich KL, Brown RW. Peeters LD, et al. Psychopharmacology (Berl). 2023 Jul;240(7):1453-1464. doi: 10.1007/s00213-023-06379-7. Epub 2023 May 9. Psychopharmacology (Berl). 2023. PMID: 37160431 Free PMC article.
References
-
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources