A Single Dose of Modified Vaccinia Ankara expressing Ebola Virus Like Particles Protects Nonhuman Primates from Lethal Ebola Virus Challenge - PubMed
- ️Mon Jan 01 2018
doi: 10.1038/s41598-017-19041-y.
Friederike Feldmann 2 , Rahul Basu 1 , Nathanael McCurley 1 , Kyle Shifflett 3 , Jackson Emanuel 3 , Michael S Hellerstein 1 , Farshad Guirakhoo 1 , Chiara Orlandi 4 , Robin Flinko 4 , George K Lewis 4 , Patrick W Hanley 2 , Heinz Feldmann 3 , Harriet L Robinson 5 , Andrea Marzi 6
Affiliations
- PMID: 29339750
- PMCID: PMC5770434
- DOI: 10.1038/s41598-017-19041-y
A Single Dose of Modified Vaccinia Ankara expressing Ebola Virus Like Particles Protects Nonhuman Primates from Lethal Ebola Virus Challenge
Arban Domi et al. Sci Rep. 2018.
Abstract
Ebola virus (EBOV), isolate Makona, was the causative agent of the West African epidemic devastating predominantly Guinea, Liberia and Sierra Leone from 2013-2016. While several experimental vaccine and treatment approaches have been accelerated through human clinical trials, there is still no approved countermeasure available against this disease. Here, we report the construction and preclinical efficacy testing of a novel recombinant modified vaccinia Ankara (MVA)-based vaccine expressing the EBOV-Makona glycoprotein GP and matrix protein VP40 (MVA-EBOV). GP and VP40 form EBOV-like particles and elicit protective immune responses. In this study, we report 100% protection against lethal EBOV infection in guinea pigs after prime/boost vaccination with MVA-EBOV. Furthermore, this MVA-EBOV protected macaques from lethal disease after a single dose or prime/boost vaccination. The vaccine elicited a variety of antibody responses to both antigens, including neutralizing antibodies and antibodies with antibody-dependent cellular cytotoxic activity specific for GP. This is the first report that a replication-deficient MVA vector can confer full protection against lethal EBOV challenge after a single dose vaccination in macaques.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
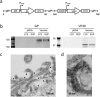
MVA-EBOV vaccine construction and expression. (a) Schematic of MVA-EBOV vaccine expressing EBOV-like particles. I8R and G1L, conserved vaccinia sequences flanking the insertion site for gp; A50R and B1R, conserved sequences flanking the insertion site for vp40. PmH5, modified early late H5 vaccinia promoter. Numbers indicate positions in the MVA genome which is abbreviated and not to scale. (b) Western blot showing expression of EBOV GP (monoclonal Ab c6D8) and VP40 (polyclonal Ab) in 293 T cell lysates (LYS) and supernatants (SUP). Molecular weight markers are indicated to the left of blots. (c) Thin section electron micrograph showing the expression of EBOV-like particles. Arrows in (c) show examples of immunogold staining for GP. (d) Magnified thin section electron micrograph showing the immunogold staining of GP (black dots) without arrows.

MVA-EBOV protection study in guinea pigs. (a) Survival curves for animals primed at week 0 and boosted at week 4 with MVA-EBOV or MVAwt. Controls are unvaccinated animals. At 8 weeks (day 0) all animals were challenged with the EBOV-Mayinga-based GPA-EBOV. (b) Body weight changes post challenge. (c) Anti-GP-specific Ab measured pre-immunization (pre), at 4 weeks after the prime (Wk4) and 2 weeks after the boost (Wk6). (d) Neutralizing Ab measured as 50% focus reduction neutralization titer (FRNT50). Significance levels are indicated as follows: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***) and p < 0.0001 (****).
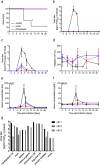
MVA-EBOV protection study in rhesus macaques. (a) Survival curves of animals receiving prime or prime/boost with MVA-EBOV or MVAwt (control) and challenge with EBOV-Makona on day 0. Viremia levels determined in whole blood samples over time by (b) virus titration and (c) RT-qPCR. (d) Counts of platelets in macaque whole blood samples over time. Levels of (e) aspartate amino transferase (AST) and (f) blood urea nitrogen (BUN) in the serum of challenged macaques over time. (g) EBOV titers in selected tissue samples of MVAwt control animals at the time of euthanasia. Significance levels are indicated as follows: p < 0.05 (*), and p < 0.01 (**). All other results are not statistically significant.
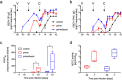
Humoral responses after vaccination and challenge. (a) EBOV GP-specific and (b) EBOV VP40-specific IgG during the immunization and challenge phases of the study. Arrows indicate days of vaccination (V; day −56, −28) and challenge (C, day 0). (c) Neutralizing titers on day 0 and 42 of the study. (d) ADCC activity pre-immunization (day −28 for prime group and day −56 for prime/boost group) and on day −7 (pre-challenge). Significance level is indicated as p < 0.05 (*); all other results are not statistically significant.
Similar articles
-
Recombinant Modified Vaccinia Virus Ankara Generating Ebola Virus-Like Particles.
Schweneker M, Laimbacher AS, Zimmer G, Wagner S, Schraner EM, Wolferstätter M, Klingenberg M, Dirmeier U, Steigerwald R, Lauterbach H, Hochrein H, Chaplin P, Suter M, Hausmann J. Schweneker M, et al. J Virol. 2017 May 12;91(11):e00343-17. doi: 10.1128/JVI.00343-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331098 Free PMC article.
-
Lázaro-Frías A, Gómez-Medina S, Sánchez-Sampedro L, Ljungberg K, Ustav M, Liljeström P, Muñoz-Fontela C, Esteban M, García-Arriaza J. Lázaro-Frías A, et al. J Virol. 2018 May 14;92(11):e00363-18. doi: 10.1128/JVI.00363-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514907 Free PMC article.
-
Ebolavirus Glycoprotein Fc Fusion Protein Protects Guinea Pigs against Lethal Challenge.
Konduru K, Shurtleff AC, Bradfute SB, Nakamura S, Bavari S, Kaplan G. Konduru K, et al. PLoS One. 2016 Sep 13;11(9):e0162446. doi: 10.1371/journal.pone.0162446. eCollection 2016. PLoS One. 2016. PMID: 27622456 Free PMC article.
-
From bench to almost bedside: the long road to a licensed Ebola virus vaccine.
Wong G, Mendoza EJ, Plummer FA, Gao GF, Kobinger GP, Qiu X. Wong G, et al. Expert Opin Biol Ther. 2018 Feb;18(2):159-173. doi: 10.1080/14712598.2018.1404572. Epub 2017 Nov 17. Expert Opin Biol Ther. 2018. PMID: 29148858 Free PMC article. Review.
-
Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus.
Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, Kumar D, Ramakrishnan MA, Malik YS, Singh R, Malik SVS, Singh RK, Chaicumpa W. Dhama K, et al. Front Immunol. 2018 Aug 10;9:1803. doi: 10.3389/fimmu.2018.01803. eCollection 2018. Front Immunol. 2018. PMID: 30147687 Free PMC article. Review.
Cited by
-
Ilinykh PA, Huang K, Gunn BM, Kuzmina NA, Kedarinath K, Jurado-Cobena E, Zhou F, Subramani C, Hyde MA, Velazquez JV, Williamson LE, Gilchuk P, Carnahan RH, Alter G, Crowe JE Jr, Bukreyev A. Ilinykh PA, et al. Commun Biol. 2024 Jul 17;7(1):871. doi: 10.1038/s42003-024-06556-0. Commun Biol. 2024. PMID: 39020082 Free PMC article.
-
An update on nonhuman primate usage for drug and vaccine evaluation against filoviruses.
de La Vega MA, Xiii A, Massey S, Spengler JR, Kobinger GP, Woolsey C. de La Vega MA, et al. Expert Opin Drug Discov. 2024 Oct;19(10):1185-1211. doi: 10.1080/17460441.2024.2386100. Epub 2024 Aug 18. Expert Opin Drug Discov. 2024. PMID: 39090822 Review.
-
Yoshikawa T, Taniguchi S, Kato H, Iwata-Yoshikawa N, Tani H, Kurosu T, Fujii H, Omura N, Shibamura M, Watanabe S, Egawa K, Inagaki T, Sugimoto S, Phanthanawiboon S, Harada S, Yamada S, Fukushi S, Morikawa S, Nagata N, Shimojima M, Saijo M. Yoshikawa T, et al. PLoS Pathog. 2021 Feb 3;17(2):e1008859. doi: 10.1371/journal.ppat.1008859. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33534867 Free PMC article.
-
Pan-ebolavirus protective therapy by two multifunctional human antibodies.
Gilchuk P, Murin CD, Cross RW, Ilinykh PA, Huang K, Kuzmina N, Borisevich V, Agans KN, Geisbert JB, Zost SJ, Nargi RS, Sutton RE, Suryadevara N, Bombardi RG, Carnahan RH, Bukreyev A, Geisbert TW, Ward AB, Crowe JE Jr. Gilchuk P, et al. Cell. 2021 Oct 28;184(22):5593-5607.e18. doi: 10.1016/j.cell.2021.09.035. Cell. 2021. PMID: 34715022 Free PMC article.
-
Furuyama W, Marzi A. Furuyama W, et al. Microorganisms. 2020 Oct 6;8(10):1535. doi: 10.3390/microorganisms8101535. Microorganisms. 2020. PMID: 33036194 Free PMC article.
References
-
- CDC. Outbreaks Chronology: Ebola Virus Disease, http://www.cdc.gov/vhf/ebola/outbreaks/history/chronology.html (2015).
-
- CDC. Chronology of Marburg Hemorrhagic Fever Outbreaks, http://www.cdc.gov/vhf/marburg/resources/outbreak-table.html (2015).
-
- WHO. WHO Ebola Situation Report (2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical