Cell-cell adhesion interface: orthogonal and parallel forces from contraction, protrusion, and retraction - PubMed
- ️Mon Jan 01 2018
Review
Cell-cell adhesion interface: orthogonal and parallel forces from contraction, protrusion, and retraction
Vivian W Tang. F1000Res. 2018.
Abstract
The epithelial lateral membrane plays a central role in the integration of intercellular signals and, by doing so, is a principal determinant in the emerging properties of epithelial tissues. Mechanical force, when applied to the lateral cell-cell interface, can modulate the strength of adhesion and influence intercellular dynamics. Yet the relationship between mechanical force and epithelial cell behavior is complex and not completely understood. This commentary aims to provide an investigative look at the usage of cellular forces at the epithelial cell-cell adhesion interface.
Keywords: actin; adhesion; cell-cell; contraction; epithelial; force; intercellular; lateral membrane; protrusion; retraction.
Conflict of interest statement
No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures

( A) Actin arrangement on the apical, lateral, and basal membranes of the epithelial cell is illustrated at the mesoscopic cellular level. ( B) Connectivity between cell–cell interface and the actin cytoskeleton is illustrated at the macroscopic multi-cellular level.
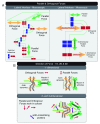
( A) Parallel and orthogonal forces are shown at the macroscopic multi-cellular (left panel) and mesoscopic cellular (right panel) scales. Cell–cell adhesions at the tip of a lateral membrane protrusion experience orthogonal force, whereas cell–cell adhesions at the trunk of the same membrane protrusion experience parallel force (right panel). ( B) Forces in one, two, and three dimensions can be generated through spatial organization of actin dynamics and actomyosin activities. Orthogonal and parallel one-dimensional forces can be generated with actin filaments arranged orthogonal and parallel to the plasma membrane, respectively (upper panel). Orthogonal and parallel two- and three-dimensional forces can be generated with a cross-linked actin network to support processes on the lateral cell–cell adhesion interface (lower panel).

( A) Pulling on adhesions and membrane can break intra-molecular and inter-molecular bonds, cause movement of adhesions on the membrane, and create undulations on the cell–cell adhesion interface. ( B) Pushing parallel to the lateral membrane can disengage and disperse adhesions, resulting in mixing of the membrane components. Pushing orthogonal to the lateral membrane can increase cell–cell contact area and time to enhance adhesion engagement.
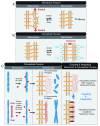
( A) Stretching the membrane increases membrane tension at the mesoscopic lateral membrane level and can enhance intercellular interactions at the microscopic molecular level. ( B) Pulling orthogonal to the membrane increases intercellular tension on the mesoscopic lateral membrane level and can enhance tension at cell–cell adhesion molecules at the microscopic molecular level. ( C) Pulling actin filament alters actin dynamics and decreases cofilin severing at the microscopic molecular level (left panel). Changing the length distribution and organization of actin filaments alters cytoskeletal tension, ultimately affecting the compressibility and rigidity of cortical actin associated with the lateral membrane at the mesoscopic lateral membrane level (middle panel). Coupling membrane and cytoskeleton tension to adhesion proteins allows integration of intercellular, membrane, cytoskeletal forces on the lateral cell–cell adhesion interface (right panel).
Similar articles
-
Teng X, Qin L, Le Borgne R, Toyama Y. Teng X, et al. Development. 2017 Jan 1;144(1):95-105. doi: 10.1242/dev.139865. Epub 2016 Nov 25. Development. 2017. PMID: 27888195
-
Equilibrium mechanics of monolayered epithelium.
Derganc J, Svetina S, Zeks B. Derganc J, et al. J Theor Biol. 2009 Oct 7;260(3):333-9. doi: 10.1016/j.jtbi.2009.06.021. Epub 2009 Jul 1. J Theor Biol. 2009. PMID: 19576229
-
Barnhart E, Lee KC, Allen GM, Theriot JA, Mogilner A. Barnhart E, et al. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5045-50. doi: 10.1073/pnas.1417257112. Epub 2015 Apr 6. Proc Natl Acad Sci U S A. 2015. PMID: 25848042 Free PMC article.
-
Patterns in space: coordinating adhesion and actomyosin contractility at E-cadherin junctions.
Wu SK, Yap AS. Wu SK, et al. Cell Commun Adhes. 2013 Dec;20(6):201-12. doi: 10.3109/15419061.2013.856889. Epub 2013 Nov 8. Cell Commun Adhes. 2013. PMID: 24205985 Review.
-
Coupling membrane protrusion and cell adhesion.
DeMali KA, Burridge K. DeMali KA, et al. J Cell Sci. 2003 Jun 15;116(Pt 12):2389-97. doi: 10.1242/jcs.00605. J Cell Sci. 2003. PMID: 12766185 Review.
Cited by
-
Structural Elements of the Biomechanical System of Soft Tissue.
Smit HJ, Strong P. Smit HJ, et al. Cureus. 2020 Apr 30;12(4):e7895. doi: 10.7759/cureus.7895. Cureus. 2020. PMID: 32368430 Free PMC article. Review.
-
De Luca M, Mandala M, Rose G. De Luca M, et al. Mech Ageing Dev. 2021 Jul;197:111522. doi: 10.1016/j.mad.2021.111522. Epub 2021 Jun 18. Mech Ageing Dev. 2021. PMID: 34147549 Free PMC article. Review.
-
Actin cytoskeleton dynamics during mucosal inflammation: a view from broken epithelial barriers.
Lechuga S, Ivanov AI. Lechuga S, et al. Curr Opin Physiol. 2021 Feb;19:10-16. doi: 10.1016/j.cophys.2020.06.012. Epub 2020 Jun 30. Curr Opin Physiol. 2021. PMID: 32728653 Free PMC article.
-
Intrinsic cell rheology drives junction maturation.
Sri-Ranjan K, Sanchez-Alonso JL, Swiatlowska P, Rothery S, Novak P, Gerlach S, Koeninger D, Hoffmann B, Merkel R, Stevens MM, Sun SX, Gorelik J, Braga VMM. Sri-Ranjan K, et al. Nat Commun. 2022 Aug 17;13(1):4832. doi: 10.1038/s41467-022-32102-9. Nat Commun. 2022. PMID: 35977954 Free PMC article.
-
Teo JL, Tomatis VM, Coburn L, Lagendijk AK, Schouwenaar IM, Budnar S, Hall TE, Verma S, McLachlan RW, Hogan BM, Parton RG, Yap AS, Gomez GA. Teo JL, et al. Mol Biol Cell. 2020 Nov 1;31(23):2557-2569. doi: 10.1091/mbc.E20-01-0084. Epub 2020 Sep 9. Mol Biol Cell. 2020. PMID: 32903148 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources