Host Cell Restriction Factors of Paramyxoviruses and Pneumoviruses - PubMed
- ️Wed Jan 01 2020
Review
Host Cell Restriction Factors of Paramyxoviruses and Pneumoviruses
Rubaiyea Farrukee et al. Viruses. 2020.
Abstract
The paramyxo- and pneumovirus family includes a wide range of viruses that can cause respiratory and/or systemic infections in humans and animals. The significant disease burden of these viruses is further exacerbated by the limited therapeutics that are currently available. Host cellular proteins that can antagonize or limit virus replication are therefore a promising area of research to identify candidate molecules with the potential for host-targeted therapies. Host proteins known as host cell restriction factors are constitutively expressed and/or induced in response to virus infection and include proteins from interferon-stimulated genes (ISGs). Many ISG proteins have been identified but relatively few have been characterized in detail and most studies have focused on studying their antiviral activities against particular viruses, such as influenza A viruses and human immunodeficiency virus (HIV)-1. This review summarizes current literature regarding host cell restriction factors against paramyxo- and pneumoviruses, on which there is more limited data. Alongside discussion of known restriction factors, this review also considers viral countermeasures in overcoming host restriction, the strengths and limitations in different experimental approaches in studies reported to date, and the challenges in reconciling differences between in vitro and in vivo data. Furthermore, this review provides an outlook regarding the landscape of emerging technologies and tools available to study host cell restriction factors, as well as the suitability of these proteins as targets for broad-spectrum antiviral therapeutics.
Keywords: innate; interferon-stimulated gene; paramyxovirus; pneumovirus; replication; restriction factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
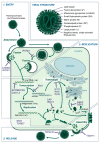
Structure and Replication of Paramyxo- and Pneumoviruses. Paramyxo- and pneumoviruses share a common structure comprising surface fusion (F) and attachment (H/HN/G) proteins (and an additional small hydrophobic (SH) protein for some) located in a host-derived lipid bilayer. Beneath this layer, the matrix (M) protein forms a shell within which is contained the ribonucleocapsid, comprising the phosphoprotein (P) and Large (L) polymerase subunit, as well as a negative-sense, single-stranded RNA genome bound by nucleoprotein (NP) in the form of ribonculeoproteins (RNPs). (1) Upon attachment to the host cell via H/HN/G proteins, paramyxovirus and pneumovirus entry is facilitated by the F protein, releasing the ribonucleocapsid into the cytosol. Pneumoviruses may additionally enter the cell via endocytosis. CH25H, IFITM1 and IFITM3 have been identified to inhibit entry of some viruses. (2) Viral RNA is then transcribed to mRNA and the viral proteins translated, with the glycoproteins (H/HN/G, F and SH) travelling through the ER to the Golgi before reaching the cell surface. Viral RNA is additionally transcribed to produce a positive-sense antigenome, which is replicated to give negative-sense genomic RNA. Restriction factors known to inhibit these stages of virus replication include, ABOBEC3G, OAS1, OAS2, MxA, IFIT1, PKR, ZAP, ISG15, GBP2, and GBP5. The exact step in the virus replication process inhibited by IDO1 and TDRD7 has not been identified. (3) After genomic replication, RNA associates with the NP to form RNPs, as well as the P and L proteins, and traffics to the cell membrane where it interacts with M protein. Once this ribonucleocapsid is assembled with the surface proteins, newly-formed virions are released by budding. The restriction factors tetherin and viperin can interfere with assembly and budding.
Similar articles
-
Shrestha N, Gall FM, Mathieu C, Hierweger MM, Brügger M, Alves MP, Vesin J, Banfi D, Kalbermatter D, Horvat B, Chambon M, Turcatti G, Fotiadis D, Riedl R, Plattet P. Shrestha N, et al. mBio. 2021 Dec 21;12(6):e0262121. doi: 10.1128/mBio.02621-21. Epub 2021 Nov 2. mBio. 2021. PMID: 34724816 Free PMC article.
-
Structural Insight into Paramyxovirus and Pneumovirus Entry Inhibition.
Aggarwal M, Plemper RK. Aggarwal M, et al. Viruses. 2020 Mar 20;12(3):342. doi: 10.3390/v12030342. Viruses. 2020. PMID: 32245118 Free PMC article. Review.
-
Paramyxovirus activation and inhibition of innate immune responses.
Parks GD, Alexander-Miller MA. Parks GD, et al. J Mol Biol. 2013 Dec 13;425(24):4872-92. doi: 10.1016/j.jmb.2013.09.015. Epub 2013 Sep 20. J Mol Biol. 2013. PMID: 24056173 Free PMC article. Review.
-
Polymerases of paramyxoviruses and pneumoviruses.
Fearns R, Plemper RK. Fearns R, et al. Virus Res. 2017 Apr 15;234:87-102. doi: 10.1016/j.virusres.2017.01.008. Epub 2017 Jan 16. Virus Res. 2017. PMID: 28104450 Free PMC article. Review.
-
Interferon-Mediated Response to Human Metapneumovirus Infection.
Uche IK, Guerrero-Plata A. Uche IK, et al. Viruses. 2018 Sep 18;10(9):505. doi: 10.3390/v10090505. Viruses. 2018. PMID: 30231515 Free PMC article. Review.
Cited by
-
TRIM16 Overexpression in HEK293T Cells Results in Cell Line-Specific Antiviral Activity.
Nigos LR, Scott NE, Brooks AG, Ait-Goughoulte M, Londrigan SL, Reading PC, Farrukee R. Nigos LR, et al. Pathogens. 2023 Jun 20;12(6):852. doi: 10.3390/pathogens12060852. Pathogens. 2023. PMID: 37375542 Free PMC article.
-
Lee AR, Park YK, Dezhbord M, Kim KH. Lee AR, et al. Viruses. 2022 Feb 11;14(2):373. doi: 10.3390/v14020373. Viruses. 2022. PMID: 35215970 Free PMC article. Review.
-
Duan Z, Shi H, Xing J, Zhang Q, Liu M. Duan Z, et al. Int J Mol Sci. 2023 Jan 4;24(2):980. doi: 10.3390/ijms24020980. Int J Mol Sci. 2023. PMID: 36674496 Free PMC article.
-
Paramyxoviruses from bats: changes in receptor specificity and their role in host adaptation.
Haas GD, Lee B. Haas GD, et al. Curr Opin Virol. 2023 Feb;58:101292. doi: 10.1016/j.coviro.2022.101292. Epub 2022 Dec 9. Curr Opin Virol. 2023. PMID: 36508860 Free PMC article. Review.
-
Afroz S, Saul S, Dai J, Surman S, Liu X, Park HS, Le Nouën C, Lingemann M, Dahal B, Coleman JR, Mueller S, Collins PL, Buchholz UJ, Munir S. Afroz S, et al. Proc Natl Acad Sci U S A. 2024 Jun 18;121(25):e2316376121. doi: 10.1073/pnas.2316376121. Epub 2024 Jun 11. Proc Natl Acad Sci U S A. 2024. PMID: 38861603 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources