Spine dynamics of PSD-95-deficient neurons in the visual cortex link silent synapses to structural cortical plasticity - PubMed
- ️Fri Jan 01 2021
Spine dynamics of PSD-95-deficient neurons in the visual cortex link silent synapses to structural cortical plasticity
Rashad Yusifov et al. Proc Natl Acad Sci U S A. 2021.
Abstract
Critical periods (CPs) are time windows of heightened brain plasticity during which experience refines synaptic connections to achieve mature functionality. At glutamatergic synapses on dendritic spines of principal cortical neurons, the maturation is largely governed by postsynaptic density protein-95 (PSD-95)-dependent synaptic incorporation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors into nascent AMPA-receptor silent synapses. Consequently, in mouse primary visual cortex (V1), impaired silent synapse maturation in PSD-95-deficient neurons prevents the closure of the CP for juvenile ocular dominance plasticity (jODP). A structural hallmark of jODP is increased spine elimination, induced by brief monocular deprivation (MD). However, it is unknown whether impaired silent synapse maturation facilitates spine elimination and also preserves juvenile structural plasticity. Using two-photon microscopy, we assessed spine dynamics in apical dendrites of layer 2/3 pyramidal neurons (PNs) in binocular V1 during ODP in awake adult mice. Under basal conditions, spine formation and elimination ratios were similar between PSD-95 knockout (KO) and wild-type (WT) mice. However, a brief MD affected spine dynamics only in KO mice, where MD doubled spine elimination, primarily affecting newly formed spines, and caused a net reduction in spine density similar to what has been observed during jODP in WT mice. A similar increase in spine elimination after MD occurred if PSD-95 was knocked down in single PNs of layer 2/3. Thus, structural plasticity is dictated cell autonomously by PSD-95 in vivo in awake mice. Loss of PSD-95 preserves hallmark features of spine dynamics in jODP into adulthood, revealing a functional link of PSD-95 for experience-dependent synapse maturation and stabilization during CPs.
Keywords: awake; plasticity; silent synapses; spine dynamics; visual cortex.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

Repeated two-photon imaging of apical dendrites of L2/3 pyramidal neurons in the primary visual cortex (V1) of awake head-fixed PSD-95 WT and KO mice during periods of normal vision (NV), monocular deprivation (MD), and after reopening the formerly deprived eye (RO). (A) Experimental timeline: a plasmid expressing eGFP or eGFP/sh95 was delivered into the left ventricle of E15.5 PSD-95 WT and KO pups and electroporated targeting V1. After implanting a chronic cranial window (∼P45), optical imaging of intrinsic signals was used to locate binocular V1 at ∼P50. Mice were then screened for sparse neuronal labeling and habituated to head restraint for 15 to 20 d. After baseline measurements (d0 to d4), a 4-d MD of the contralateral eye was started on d4, and the eye was reopened on d8. Awake two-photon imaging of dendritic spines was performed on all days indicated by the black arrows. (B) Coronal slice of an electroporated brain illustrating L2/3 pyramidal neuron-specific eGFP expression in V1. (Scale bar, 400 μm.) (C) Cortical blood vessel pattern imaged through the cranial window with an outline of binocular V1 (dashed ellipse), located by (D) retinotopic mapping using intrinsic signal optical imaging (Scale bar, 1 mm). (E) Low-magnification top view of an imaged region with sparsely eGFP-labeled L2/3 pyramidal neurons. (Scale bar, 50 μm.) (F) Average baseline spine densities (±SEM) of apical dendrites in PSD-95 WT and KO mice on d0 to d4. (G) The same dendrites were repeatedly imaged with 1-d (5 WT/4 KO: 25/21 dendrites) and 4-d (6 WT/6 KO: 40/36 dendrites) intervals during NV and MD periods. Imaging was continued 2 d (6 WT/6 KO: 33/31 dendrites) and 4 d (6 WT/6 KO: 33/30 dendrites) after RO. Spine density (normalized to average baseline) was significantly reduced in PSD-95 KO but not WT mice after 4-d MD, and the reduction persisted after reopening the eye. *P < 0.05; **P < 0.01; ***P < 0.001; ns, P > 0.05.

In V1 of adult PSD-95 KO mice, monocular deprivation induces increased spine elimination and decreased spine formation on apical dendrites of L2/3 pyramidal neurons. Experience-dependent spine dynamics of L2/3 pyramidal neurons and their quantification. (A) Representative examples of dendrites from PSD-95 WT (n = 6) and (B) KO mice (n = 6) on d0 (Left), d4 (Middle), and d8 (Right): red arrows mark eliminated and green arrows mark newly formed spines during 4-d intervals. (Scale bar, 5 μm). (C and D) Mean (±SD) spine elimination and (F and G) spine formation ratios during 1-d (C and F; 5 WT/4 KO: 25/21 dendrites) and 4-d (D and G; 6 WT/6 KO: 40/36 dendrites) intervals, calculated as R = Nelmn or Nform/(Ninitial + Nfinal). (E) MD-induced change Δ = RMD - RNV in spine elimination and (H) formation ratios (mean ± SEM). *P < 0.05; **P < 0.01; ***P < 0.001; ns, P > 0.05.
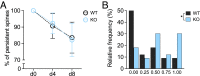
Newly formed spines in PSD-95 KO mice are more likely to be eliminated during MD. MD-induced changes in persistent (lifetime > 4 d) and newly formed spine populations in PSD-95 KO and WT mice. (A) Percentage of persistent spines (mean ± SD) present from d0 throughout NV and MD periods. (B) Histogram depicts relative frequency of PSD-95 WT and KO dendrites binned according to the fractions of newly formed spines that were eliminated during 4-d MD (0.00 = none eliminated; 1.00 = all new spines eliminated during MD). Note that in PSD-95 KO mice, the frequency histogram is skewed toward more spine elimination during MD. *P < 0.05.

Dendritic spine formation of L2/3 pyramidal neurons returns to baseline values in PSD-95 KO mice, while no significant changes happen in WT mice after reopening. Imaging was continued in a subset of dendrites described in Fig. 2, 4 d (6 WT/6 KO: 33/30 dendrites) after reopening the MD eye. (A) Representative examples of dendrites from PSD-95 WT and (B) PSD-95 KO mice on d8 (Left) and d12 (Right): green arrows mark formed and red arrows mark eliminated spines compared to d8 (Scale bar, 5 μm). (C) Comparison of mean (±SD) spine elimination and (D) formation ratios during 4-d reopening to NV and MD periods (gray rectangle, data replotted from Fig. 2 D and G for comparison). *P < 0.05; **P < 0.01; ***P < 0.001; ns, P > 0.05.
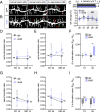
In V1 of WT mice, MD induces increased spine elimination on apical dendrites of PSD-95 knockdown L2/3 pyramidal neurons. (A) Apical dendrites of L2/3 pyramidal neurons expressing either eGFP-tagged control (gfp: 4 mice/n = 19) or (B) PSD-95 knockdown plasmids (sh95: 3 mice/n = 21) were repeatedly imaged with 1- and 4-d intervals during NV and MD periods: red arrows mark eliminated, green arrows mark formed spines during 4-d intervals. (Scale bar, 5 μm.) (C) Average baseline spine density (±SEM) during NV and net change of spine density during NV and MD periods (gray shaded area) relative to baseline (d0 to d4). (D and E) Mean (±SD) spine elimination and (G and H) spine formation ratios during (D and G) 1-d and (E and H) 4-d intervals. (F) MD-induced change (Δ = RMD - RNV) in spine elimination and (I) formation ratios (mean ± SEM). *P < 0.05; **P < 0.01; ns, P > 0.05.
Similar articles
-
An opposing function of paralogs in balancing developmental synapse maturation.
Favaro PD, Huang X, Hosang L, Stodieck S, Cui L, Liu YZ, Engelhardt KA, Schmitz F, Dong Y, Löwel S, Schlüter OM. Favaro PD, et al. PLoS Biol. 2018 Dec 26;16(12):e2006838. doi: 10.1371/journal.pbio.2006838. eCollection 2018 Dec. PLoS Biol. 2018. PMID: 30586380 Free PMC article.
-
Ocular Dominance Plasticity after Stroke Was Preserved in PSD-95 Knockout Mice.
Greifzu F, Parthier D, Goetze B, Schlüter OM, Löwel S. Greifzu F, et al. PLoS One. 2016 Mar 1;11(3):e0149771. doi: 10.1371/journal.pone.0149771. eCollection 2016. PLoS One. 2016. PMID: 26930616 Free PMC article.
-
Progressive maturation of silent synapses governs the duration of a critical period.
Huang X, Stodieck SK, Goetze B, Cui L, Wong MH, Wenzel C, Hosang L, Dong Y, Löwel S, Schlüter OM. Huang X, et al. Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):E3131-40. doi: 10.1073/pnas.1506488112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26015564 Free PMC article.
-
Silent Synapse-Based Mechanisms of Critical Period Plasticity.
Xu W, Löwel S, Schlüter OM. Xu W, et al. Front Cell Neurosci. 2020 Jul 17;14:213. doi: 10.3389/fncel.2020.00213. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32765222 Free PMC article. Review.
-
Silent synapse: A new player in visual cortex critical period plasticity.
Huang X. Huang X. Pharmacol Res. 2019 Mar;141:586-590. doi: 10.1016/j.phrs.2019.01.031. Epub 2019 Jan 16. Pharmacol Res. 2019. PMID: 30659896 Review.
Cited by
-
Thalamic regulation of ocular dominance plasticity in adult visual cortex.
Qin Y, Ahmadlou M, Suhai S, Neering P, de Kraker L, Heimel JA, Levelt CN. Qin Y, et al. Elife. 2023 Oct 5;12:RP88124. doi: 10.7554/eLife.88124. Elife. 2023. PMID: 37796249 Free PMC article.
-
Enhanced long-term potentiation in the anterior cingulate cortex of tree shrew.
Song Q, Li XH, Lu JS, Chen QY, Liu RH, Zhou SB, Zhuo M. Song Q, et al. Philos Trans R Soc Lond B Biol Sci. 2024 Jul 29;379(1906):20230240. doi: 10.1098/rstb.2023.0240. Epub 2024 Jun 10. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38853555 Free PMC article.
-
Liaci C, Camera M, Caslini G, Rando S, Contino S, Romano V, Merlo GR. Liaci C, et al. Int J Mol Sci. 2021 Jun 7;22(11):6167. doi: 10.3390/ijms22116167. Int J Mol Sci. 2021. PMID: 34200511 Free PMC article. Review.
-
Yook Y, Lee KY, Kim E, Lizarazo S, Yu X, Tsai NP. Yook Y, et al. EMBO Rep. 2024 Mar;25(3):1233-1255. doi: 10.1038/s44319-024-00090-0. Epub 2024 Feb 27. EMBO Rep. 2024. PMID: 38413732 Free PMC article.
-
Li A, Huang CJ, Gu KP, Huang Y, Huang YQ, Zhang H, Lin JP, Liu YF, Yang Y, Yao YX. Li A, et al. Sci Rep. 2022 Oct 12;12(1):17114. doi: 10.1038/s41598-022-21488-7. Sci Rep. 2022. PMID: 36224339 Free PMC article.
References
-
- Wiesel T. N., Hubel D. H., Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017 (1963). - PubMed
-
- Zuo Y., Lin A., Chang P., Gan W. B., Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46, 181–189 (2005). - PubMed
-
- Holtmaat A. J., et al. ., Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45, 279–291 (2005). - PubMed
-
- Trachtenberg J. T., et al. ., Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420, 788–794 (2002). - PubMed
-
- Lendvai B., Stern E. A., Chen B., Svoboda K., Experience-dependent plasticity of dendritic spines in the developing rat barrel cortex in vivo. Nature 404, 876–881 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous