Physiologically based metformin pharmacokinetics model of mice and scale-up to humans for the estimation of concentrations in various tissues - PubMed
- ️Fri Jan 01 2021
Randomized Controlled Trial
Physiologically based metformin pharmacokinetics model of mice and scale-up to humans for the estimation of concentrations in various tissues
Darta Maija Zake et al. PLoS One. 2021.
Abstract
Metformin is the primary drug for type 2 diabetes treatment and a promising candidate for other disease treatment. It has significant deviations between individuals in therapy efficiency and pharmacokinetics, leading to the administration of an unnecessary overdose or an insufficient dose. There is a lack of data regarding the concentration-time profiles in various human tissues that limits the understanding of pharmacokinetics and hinders the development of precision therapies for individual patients. The physiologically based pharmacokinetic (PBPK) model developed in this study is based on humans' known physiological parameters (blood flow, tissue volume, and others). The missing tissue-specific pharmacokinetics parameters are estimated by developing a PBPK model of metformin in mice where the concentration time series in various tissues have been measured. Some parameters are adapted from human intestine cell culture experiments. The resulting PBPK model for metformin in humans includes 21 tissues and body fluids compartments and can simulate metformin concentration in the stomach, small intestine, liver, kidney, heart, skeletal muscle adipose, and brain depending on the body weight, dose, and administration regimen. Simulations for humans with a bodyweight of 70kg have been analyzed for doses in the range of 500-1500mg. Most tissues have a half-life (T1/2) similar to plasma (3.7h) except for the liver and intestine with shorter T1/2 and muscle, kidney, and red blood cells that have longer T1/2. The highest maximal concentrations (Cmax) turned out to be in the intestine (absorption process) and kidney (excretion process), followed by the liver. The developed metformin PBPK model for mice does not have a compartment for red blood cells and consists of 20 compartments. The developed human model can be personalized by adapting measurable values (tissue volumes, blood flow) and measuring metformin concentration time-course in blood and urine after a single dose of metformin. The personalized model can be used as a decision support tool for precision therapy development for individuals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
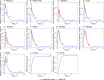
Venous plasma (A), portal vein (B), small intestine (C), liver (D), kidney (E), heart (F), muscle (G), adipose (H), brain (I), feces (J) and urine (K) following a single PO 50 mg/kg dose in mice. The red marks represent the experimental data’s concentration-time profiles with error bars representing standard deviation [1] and the blue lines represent the model simulations.
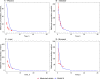
Venous plasma (A), small intestine (B), liver (C), and stomach (D) following a single intravenous 50 mg/kg dose in mice. The red marks represent the experimental data’s concentration-time profiles with error bars representing standard deviation [1] and the blue lines represent the model simulations.
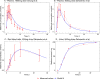
Plasma (A) following a single 1000mg PO dose in humans [36] and simulations for plasma (B), red blood cells (C), and urine (D) following a single 500mg PO dose in humans [20]. The red marks represent the experimental data’s concentration-time profiles, where the red error bars represent the standard deviation, and the blue lines represent the model simulations.
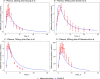
The red marks represent the experimental data’s concentration-time profiles from four different datasets, the error bars represent standard deviation, and the blue lines represent the model simulations.
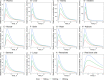
Plasma (A), liver (B), kidney (C), intestine (D), muscle (E), brain (F), heart (G), adipose (H), stomach (I), lungs (J), the remainder (K), red blood cells (L)—following a single PO dose of 500mg, 1000mg and 1500mg metformin hydrochloride in humans. The red lines represent the concentration-time profiles of the model simulations of the 500mg dose, the green lines represent model simulations for the 1000mg dose and the blue lines represent model simulations for the 1500mg dose.
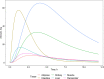
Red color curves represent adipose tissues, green–kidney, dark blue- muscle, yellow–intestine, light blue–liver, pink–remainder.
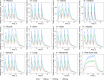
Plasma (A), liver (B), kidney (C), intestine (D), muscle (E), brain (F), heart (G), adipose (H), stomach (I), lungs (J), the remainder (K), red blood cells (L)—following four PO doses of 500mg, 1000mg and 1500mg in humans at 0, 12, 24 and 36h in humans. The red lines represent the concentration-time profiles of the model simulations of the 500mg dose, the green lines represent model simulations for the 1000mg dose and the blue lines represent model simulations for the 1500mg dose.
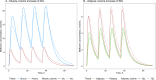
Metformin concentrations in A—muscle and plasma at normal muscle volume (28L) and increased muscle volume 48L and B—adipose and plasma at normal adipose volume (15L) and increased adipose volume 75L.
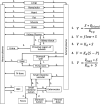
V–the reaction rate, S–the concentration of metformin at substrate side, P- the concentration of metformin at product side, Qblood−the flow to a particular compartment, Kt:p—tissue:plasma partition (Kt:p) coefficients, Kd—the non-saturable component of transport, Vmax—the maximal velocity, Km—the Michaelis-Menten constant. Red blood cells (RBC) compartment (dashed line) is used only in the human model.
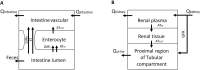
A: Intestinal structure, where Qintestine−blood flow to the small intestine, ST—saturable transport through paracellular space, AT–active transport, Diffc−diffusion into cells, DiffPC−paracellular diffusion, ATIN−active transport into cells by OCT3 and PAMAT transporters, ATOUT—active transport out of cells by OCT1 transporters; B: Renal structure, where Qkidney−renal blood flow, Qurine−urine flow, GFR—glomerular filtration rate, ATIN−active transport into cells by OCT2 transporters, ATOUT—active transport out of cells by MATE1, MATE2-K and OCT1 transporters.
Similar articles
-
Population exposure-response modeling of metformin in patients with type 2 diabetes mellitus.
Hong Y, Rohatagi S, Habtemariam B, Walker JR, Schwartz SL, Mager DE. Hong Y, et al. J Clin Pharmacol. 2008 Jun;48(6):696-707. doi: 10.1177/0091270008316884. Epub 2008 Mar 27. J Clin Pharmacol. 2008. PMID: 18372428 Clinical Trial.
-
Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, Timmins P, Graham GG, Furlong TJ, Greenfield JR, Williams KM, Day RO. Duong JK, et al. Clin Pharmacokinet. 2013 May;52(5):373-84. doi: 10.1007/s40262-013-0046-9. Clin Pharmacokinet. 2013. PMID: 23475568 Clinical Trial.
-
Duong JK, Kroonen MYAM, Kumar SS, Heerspink HL, Kirkpatrick CM, Graham GG, Williams KM, Day RO. Duong JK, et al. Eur J Clin Pharmacol. 2017 Aug;73(8):981-990. doi: 10.1007/s00228-017-2251-1. Epub 2017 Apr 28. Eur J Clin Pharmacol. 2017. PMID: 28451709
-
Clinical pharmacokinetics of metformin.
Scheen AJ. Scheen AJ. Clin Pharmacokinet. 1996 May;30(5):359-71. doi: 10.2165/00003088-199630050-00003. Clin Pharmacokinet. 1996. PMID: 8743335 Review.
-
Metformin Biodistribution: A Key to Mechanisms of Action?
Sundelin E, Jensen JB, Jakobsen S, Gormsen LC, Jessen N. Sundelin E, et al. J Clin Endocrinol Metab. 2020 Nov 1;105(11):dgaa332. doi: 10.1210/clinem/dgaa332. J Clin Endocrinol Metab. 2020. PMID: 32480406 Review.
Cited by
-
Villa-Fernández E, García AV, Fernández-Fernández A, García-Villarino M, Ares-Blanco J, Pujante P, González-Vidal T, Fraga MF, Torre EM, Delgado E, Lambert C. Villa-Fernández E, et al. Int J Mol Sci. 2024 Feb 24;25(5):2637. doi: 10.3390/ijms25052637. Int J Mol Sci. 2024. PMID: 38473884 Free PMC article.
-
Metformin Transport Rates Between Plasma and Red Blood Cells in Humans.
Kurlovics J, Zake DM, Zaharenko L, Berzins K, Klovins J, Stalidzans E. Kurlovics J, et al. Clin Pharmacokinet. 2022 Jan;61(1):133-142. doi: 10.1007/s40262-021-01058-2. Epub 2021 Jul 26. Clin Pharmacokinet. 2022. PMID: 34309806 Free PMC article.
-
Radioprotective effect of the anti-diabetic drug metformin.
Siteni S, Barron S, Luitel K, Shay JW. Siteni S, et al. PLoS One. 2024 Jul 23;19(7):e0307598. doi: 10.1371/journal.pone.0307598. eCollection 2024. PLoS One. 2024. PMID: 39042641 Free PMC article.
-
Meligi NM, Dyab AKF, Paunov VN. Meligi NM, et al. Pharmaceutics. 2021 Jul 9;13(7):1048. doi: 10.3390/pharmaceutics13071048. Pharmaceutics. 2021. PMID: 34371742 Free PMC article.
-
Scheidemantle G, Duan L, Lodge M, Cummings MJ, Hilovsky D, Pham E, Wang X, Kennedy A, Liu X. Scheidemantle G, et al. Metabolomics. 2024 May 9;20(3):53. doi: 10.1007/s11306-024-02113-2. Metabolomics. 2024. PMID: 38722395 Free PMC article.
References
-
- Nathan DM, Buse JB, Davidson MB, Heine RJ, Holman RR, Sherwin R, et al.. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2006;29: 1963–1972. 10.2337/dc06-9912 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
DMZ, JK, VK, and ES are funded by Latvian Council of Science, Grant Number: LZP‐2018/2‐0088.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases