Interconnection between Cardiac Cachexia and Heart Failure-Protective Role of Cardiac Obesity - PubMed
- ️Sat Jan 01 2022
Review
Interconnection between Cardiac Cachexia and Heart Failure-Protective Role of Cardiac Obesity
María Elena Soto et al. Cells. 2022.
Abstract
Cachexia may be caused by congestive heart failure, and it is then called cardiac cachexia, which leads to increased morbidity and mortality. Cardiac cachexia also worsens skeletal muscle degradation. Cardiac cachexia is the loss of edema-free muscle mass with or without affecting fat tissue. It is mainly caused by a loss of balance between protein synthesis and degradation, or it may result from intestinal malabsorption. The loss of balance in protein synthesis and degradation may be the consequence of altered endocrine mediators such as insulin, insulin-like growth factor 1, leptin, ghrelin, melanocortin, growth hormone and neuropeptide Y. In contrast to many other health problems, fat accumulation in the heart is protective in this condition. Fat in the heart can be divided into epicardial, myocardial and cardiac steatosis. In this review, we describe and discuss these topics, pointing out the interconnection between heart failure and cardiac cachexia and the protective role of cardiac obesity. We also set the basis for possible screening methods that may allow for a timely diagnosis of cardiac cachexia, since there is still no cure for this condition. Several therapeutic procedures are discussed including exercise, nutritional proposals, myostatin antibodies, ghrelin, anabolic steroids, anti-inflammatory substances, beta-adrenergic agonists, medroxyprogesterone acetate, megestrol acetate, cannabinoids, statins, thalidomide, proteasome inhibitors and pentoxifylline. However, to this date, there is no cure for cachexia.
Keywords: adipose tissue; cardiac cachexia; cardiac fat tissue; heart failure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
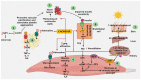
Events and processes generated by cardiac cachexia and heart failure and interrelation between different organs (skeletal muscle, heart, adipose tissue, endothelial cells, kidney, and liver) and their influence on the appearance of cachexia. (1) Leptin promotes a prothrombotic state. (2) Heart failure leads to alterations in the synthesis and degradation of cardiac proteins. (3) Leptin promotes an inflammatory environment. (4) Impaired insulin sensitivity as a result of heart failure. (5) The decrease in insulin sensitivity as a consequence of cachexia favors endothelial dysfunction. (6) Muscle wasting as a result of increased UPS and an inflammatory environment due to cachexia. (7,8) Vitamin D and VDR deficiency affect muscle structure. Abbreviations: Arg = Arginine, AR = Adrenaline receptor, cGMP = Cyclic guanosine monophosphate, eNOS = Endothelial nitric oxide synthase, IL = Interleukin, IR = Insulin receptor, LR = Leptin receptor, NO = Nitric oxide, NOX = Nicotinamide adenine dinucleotide phosphate oxidase, NPY = Neuropeptide Y, PIP2 = Phosphatidylinositol biphosphate, IP3 = Inositol triphosphate, ROS = Reactive oxygen species, SR = Sarcoplasmic reticulum, TNFα = Tumor necrosis factor alpha, UPS = Ubiquitin–protease system, VDR = Vitamin D receptor.
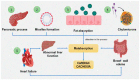
Cardiac cachexia, malabsorption syndrome and factors that favor them. (1) Hydrolysis of dietary triglyceride by pancreatic lipase. (2) The resulting fatty acids and monoglycerides then combine with bile salts from the liver to form micelles. (3) Dietary fats are absorbed across the mucous membrane into the intestinal cell. (4) The triglycerides are resynthesized and then incorporated into chylomicrons and transferred to the lymphatic system. (5) Heart failure generates alterations in hepatic functioning and bowel wall edema that favor malabsorption syndrome and, consequently, cardiac cachexia.
Similar articles
-
Cardiac cachexia: a systematic overview.
von Haehling S, Lainscak M, Springer J, Anker SD. von Haehling S, et al. Pharmacol Ther. 2009 Mar;121(3):227-52. doi: 10.1016/j.pharmthera.2008.09.009. Epub 2008 Nov 14. Pharmacol Ther. 2009. PMID: 19061914 Review.
-
[Chronic heart failure and cachexia: role of endocrine system].
Dei Cas A, Muoio A, Zavaroni I. Dei Cas A, et al. Minerva Cardioangiol. 2011 Dec;59(6):601-12. Epub 2009 Nov 30. Minerva Cardioangiol. 2011. PMID: 19946251 Review. Italian.
-
von Haehling S, Stepney R, Anker SD. von Haehling S, et al. Int J Cardiol. 2010 Oct 29;144(3):347-9. doi: 10.1016/j.ijcard.2010.05.042. Epub 2010 Jun 19. Int J Cardiol. 2010. PMID: 20561693
-
Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting.
Gullett NP, Hebbar G, Ziegler TR. Gullett NP, et al. Am J Clin Nutr. 2010 Apr;91(4):1143S-1147S. doi: 10.3945/ajcn.2010.28608E. Epub 2010 Feb 17. Am J Clin Nutr. 2010. PMID: 20164318 Free PMC article. Review.
-
The wasting continuum in heart failure: from sarcopenia to cachexia.
von Haehling S. von Haehling S. Proc Nutr Soc. 2015 Nov;74(4):367-77. doi: 10.1017/S0029665115002438. Epub 2015 Aug 12. Proc Nutr Soc. 2015. PMID: 26264581 Review.
Cited by
-
Desai A, Sharma S, Abuah N, Jang J, Desai S, Paghdhar S, Goswami RM. Desai A, et al. Front Cardiovasc Med. 2024 Jul 24;11:1376645. doi: 10.3389/fcvm.2024.1376645. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39114558 Free PMC article.
-
Soto ME, Pérez-Torres I, Rubio-Ruiz ME, Cano-Martínez A, Manzano-Pech L, Guarner-Lans V. Soto ME, et al. Int J Mol Sci. 2023 Feb 25;24(5):4534. doi: 10.3390/ijms24054534. Int J Mol Sci. 2023. PMID: 36901968 Free PMC article. Review.
-
Cardiac wasting in patients with cancer.
Anker MS, Rashid AM, Butler J, Khan MS. Anker MS, et al. Basic Res Cardiol. 2025 Feb;120(1):25-34. doi: 10.1007/s00395-024-01079-5. Epub 2024 Sep 23. Basic Res Cardiol. 2025. PMID: 39311910 Free PMC article. Review.
-
Balletti A, De Biase N, Del Punta L, Filidei F, Armenia S, Masi F, Di Fiore V, Mazzola M, Bacca A, Dini FL, Taddei S, Masi S, Pugliese NR. Balletti A, et al. Diagnostics (Basel). 2023 Feb 20;13(4):790. doi: 10.3390/diagnostics13040790. Diagnostics (Basel). 2023. PMID: 36832278 Free PMC article.
References
-
- Krysztofiak H., Wleklik M., Migaj J., Dudek M., Uchmanowicz I., Lisiak M., Kubielas G., Straburzyńska-Migaj E., Lesiak M., Kałużna-Oleksy M. Cardiac Cachexia: A Well-known but challenging complication of heart failure. Clin. Interv. Aging. 2020;5:2041–2051. doi: 10.2147/CIA.S273967. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical