A purified diet affects intestinal epithelial proliferation and barrier functions through gut microbial alterations - PubMed
- ️Mon Jan 01 2024
. 2024 Apr 3;36(5):223-240.
doi: 10.1093/intimm/dxae003.
Kisara M Hattori 1 , Kazuaki Nakata 2 , Takuma Okawa 1 2 , Seiga Komiyama 1 , Yusuke Kinashi 1 , Yuma Kabumoto 1 , Yuria Kaneko 1 2 , Motoyoshi Nagai 1 2 , Tomoko Shindo 3 , Nobuko Moritoki 3 , Yuki I Kawamura 2 , Taeko Dohi 1 , Daisuke Takahashi 1 , Shunsuke Kimura 1 , Koji Hase 1 4 5
Affiliations
- PMID: 38262747
- PMCID: PMC10989658
- DOI: 10.1093/intimm/dxae003
A purified diet affects intestinal epithelial proliferation and barrier functions through gut microbial alterations
Hiroaki Shiratori et al. Int Immunol. 2024.
Abstract
The gut microbiota plays a crucial role in maintaining epithelial barrier function. Although multiple studies have demonstrated the significance of dietary factors on the gut microbiota and mucosal barrier function, the impact of a purified diet, which has long been used in various animal experiments, on intestinal homeostasis remains to be elucidated. Here, we compared the impact of two different types of diets, a crude diet and an AIN-93G-formula purified diet, on epithelial integrity and the gut microbiota. Purified diet-fed mice exhibited shorter villi and crypt lengths and slower epithelial turnover, particularly in the ileum. In addition, antimicrobial products, including REG3γ, were substantially decreased in purified diet-fed mice. Purified diet feeding also suppressed α1,2-fucosylation on the epithelial surface. Furthermore, the purified diet induced metabolic rewiring to fatty acid oxidation and ketogenesis. 16S ribosomal RNA gene sequencing of the ileal contents and mucus layer revealed distinct gut microbiota compositions between the purified and crude diet-fed mice. Purified diet feeding reduced the abundance of segmented filamentous bacteria (SFB), which potently upregulate REG3γ and fucosyltransferase 2 (Fut2) by stimulating group 3 innate lymphoid cells (ILC3s) to produce IL-22. These observations illustrate that the intake of a crude diet secures epithelial barrier function by facilitating SFB colonization, whereas a purified diet insufficiently establishes the epithelial barrier, at least partly owing to the loss of SFB. Our data suggest that the influence of purified diets on the epithelial barrier integrity should be considered in experiments using purified diets.
Keywords: epithelial barrier function; epithelial metabolism; gut microbiota; segmented filamentous bacteria.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
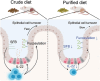

A purified diet (PD) attenuates epithelial proliferation and turnover. Three-week-old mice were fed a crude diet (CD) or a PD for 3 weeks, and the small intestine was analyzed. (A) Body weights. n ≥ 13 mice per group. (B) Length of the small intestine. n ≥ 14 mice per group. (C) Representative images of hematoxylin and eosin-staining of the ileum from PD- and CD-fed mice are shown. Images of the Swiss roll-like sections (top) and ileal epithelium (bottom). Scale bars: 2000 µm (top) or 100 µm (bottom). Scale bars: 100 µm. n ≥ 80 crypt regions from four individual mice per group were analyzed for crypt depth. n ≥ 60 villi regions from four individual mice per group for villus length. (D) Immunofluorescence images of Ki67 in the ileal crypts. Ki67+ cells only in the epithelial monolayer in the crypt region were counted. The green staining merged with nuclei was recognized as the specific signal. Scale bars: 50 µm. n ≥ 35 crypt regions from four individual mice per group were analyzed. (E) Representative images of EdU fluorescence and distance from the crypt base to the farthest EdU-labeled cells. Scale bars: 100 µm. n ≥ 30 crypt-villus regions from three individual mice per group were analyzed. (F) Representative immunofluorescence images of TFF3 and number of TFF3+ cells per ileal villus. Scale bars: 100 µm. n ≥ 35 villi regions from four individual mice per group were analyzed. (G) Immunofluorescence images of lysozyme and the number of lysozyme+ cells per crypt. Scale bars: 50 µm. n ≥ 30 crypt regions from four individual mice per group were analyzed. (H) Fluorescent images of Lgr5 and number of Lgr5+ cells per ileal crypt. Scale bars: 100 µm. n ≥ 35 crypt regions from four individual mice per group were analyzed. The data represent the mean ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001. P values were determined by unpaired t-test. CD, crude diet; PD, purified diet.
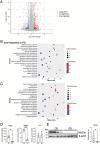
Impact of the PD on the gene expression pattern in the ileal epithelium. (A) Volcano plots comparing the ileal epithelium of CD- vs. PD-fed mice on the basis of RNA-sequencing data. n = 6. Genes up- or downregulated (Log2(fold change) > 1 or q < 0.05) are highlighted. (B, C) Gene ontology (GO) enrichment (B) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses (C) of downregulated genes in PD-fed mice. The 20 most significant GO terms are represented in the accompanying bubble plot. Bubble colors represent −log10 (P values). Bubble sizes indicate fold enrichment. (D) Relative mRNA expression of Reg3b, Reg3g, and Lyz1 in the ileal epithelium of CD- and PD-fed mice. n = 4 mice per group. (E) Representative immunoblot of the ileal epithelium from CD- and PD-fed mice for detecting REG3γ and β-actin (loading control). Band intensities were measured using densitometry. n = 4 mice per group. The data represent the mean ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001. P values were determined by unpaired t-test. CD, crude diet; PD, purified diet.

PD feeding induces metabolic rewiring to fatty acid oxidation and ketogenesis in the ileum. (A, B) GO enrichment (A) and KEGG pathway analyses (B) of upregulated genes in PD-fed mice. The 20 most significant GO terms are represented in the accompanying bubble plot. Bubble colors represent −log10 (P values). Bubble sizes indicate fold enrichment. (C) Relative mRNA expression of Ppara, Hmgcs2, Pdk4, and Fabp1 in the ileal epithelium of CD- and PD-fed mice. n = 4 mice per group. (D) Representative immunoblots of the ileal epithelium of CD- and PD-fed mice, with detection of HMGCS2, PDK4, PPARα, FABP1, and β-actin (loading control). Band intensities were measured using densitometry. n = 4 mice per group. The data represent the mean ± SD. *P < .05, **P < .01. P values were determined by unpaired t-test. CD, crude diet; PD, purified diet.
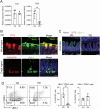
The PD suppresses epithelial fucosylation. (A) Relative mRNA expression of Fut1 and Fut2 in the ileal epithelium of CD- and PD-fed mice. n > 3 mice per group. (B) Top: Immunofluorescent images of TFF3 and UEA-1 in the villus region. Scale bars: 10 µm. Lower: Immunofluorescent images of lysozyme and UEA-1 in the crypt region. Scale bars: 40 µm. (C) Immunofluorescent images of TFF3 and UEA-1 in the ileum of CD- and PD-fed mice. Scale bars: 100 µm. (D) Representative plots of flow cytometry and percentage of UEA-1+CD24− and UEA-1+CD24+ cell subsets in the ileum of CD- and PD-fed mice. n = 4 mice per group. The data represent the mean ± SD. **P< .01. P values were determined by unpaired t-test. CD, crude diet; PD, purified diet.
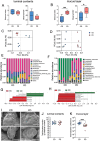
The PD alters the gut microbiota in the ileum. (A, B) α-diversities (Shannon index and observed ASVs) of the microbiota in the ileal contents (A) and ileal mucus layer (B) in CD- and PD-fed mice. (C, D) Principal coordinate analysis of weighted UniFrac distances between microbiota in the ileal contents (C) and ileal mucus layer (D). (E, F) Composition of the microbiota at the genus level in the ileal contents (E) and ileal mucus layers (F). (G, H) Discriminating taxa between CD- and PD-fed mice in the ileal contents (G) and ileal mucus layer (H) as determined using LEfSe analysis (55). (I) Scanning electron microscopy of the ileal villi from CD- or PD-fed mice. (J, K) The total bacterial load in the ileal contents (I) and ileal mucus layer (J) was analyzed using qPCR for the 16S rRNA V3–V4 region. The data represent the mean ± SD. *P < .05, **P < .01. P values were determined by unpaired t-test. CD, crude diet; PD, purified diet.
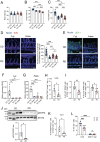
The PD affects intestinal turnover and barrier functions by reducing segmented filamentous bacteria. The effect of the PD on epithelial turnover and functions in the ileal epithelium was investigated in mice from two vendors: Fuji and Inasa. (A) Body weight, n ≥ 10 mice per group. (B) Small intestine length, n ≥ 10 mice per group. (C) Crypt depth, n ≥ 40 crypt regions from four individual mice per group were analyzed. (D) Representative images of EdU fluorescence and distance from the crypt base to the farthest EdU-labeled cells. Scale bars: 100 µm. n ≥ 30 crypt-villi regions from three individual mice per group were analyzed. (E) Immunofluorescent images of UEA-1 in the ileum. Scale bars: 100 µm. (F, G) Relative mRNA expression of Fut2 (F) and Reg3g (G) in the ileal epithelium of CD- and PD-fed mice. n = 4 mice per group. (H) The number of ILC3 (RORγt+ CD3ε−linage− cells). n ≥ 5 mice per group. (I) Percentage of IL-22+RORγt+ cells and IL-22+RORγt− cell in CD3ε−linage− cells. n ≥ 5 mice per group. (J) Representative immunoblot of the ileal epithelium of CD- and PD-fed Fuji mice, with detection of pSTAT3, STAT3, and β-actin (loading control). n = 4 mice per group. (K) Percentage of Th17 (RORγt+Foxp3−) cells in CD3ε+CD4+ cells. n ≥ 5 mice per group. (L) Percentage of IL-22+ in ILCs (CD3ε−linage−) cells and CD4+ T (CD3ε+CD4+ cells).The data represent the mean ± SD. *P < .05, **P < .01, ***P < .001, ****P < .0001. P values were determined by unpaired t-test (I–K), one-way ANOVA followed by Tukey’s multiple comparison test (A–D, F, G), or two-way ANOVA followed by Tukey’s multiple comparison test (H). CD, crude diet; ns, not significant; PD, purified diet.
Similar articles
-
Olivier S, Pochard C, Diounou H, Castillo V, Divoux J, Alcantara J, Leclerc J, Guilmeau S, Huet C, Charifi W, Varin TV, Daniel N, Foretz M, Neunlist M, Salomon BL, Ghosh P, Marette A, Rolli-Derkinderen M, Viollet B. Olivier S, et al. Mol Metab. 2021 May;47:101183. doi: 10.1016/j.molmet.2021.101183. Epub 2021 Feb 4. Mol Metab. 2021. PMID: 33548500 Free PMC article.
-
Monk JM, Lepp D, Wu W, Pauls KP, Robinson LE, Power KA. Monk JM, et al. J Nutr Biochem. 2017 Nov;49:89-100. doi: 10.1016/j.jnutbio.2017.08.002. Epub 2017 Aug 10. J Nutr Biochem. 2017. PMID: 28915390
-
Layer segmented filamentous bacteria colonize and impact gut health of broiler chickens.
Meinen-Jochum J, Skow CJ, Mellata M. Meinen-Jochum J, et al. mSphere. 2024 Nov 21;9(11):e0049224. doi: 10.1128/msphere.00492-24. Epub 2024 Oct 18. mSphere. 2024. PMID: 39422489 Free PMC article.
-
Homeostasis of the gut barrier and potential biomarkers.
Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, de Vos WM, Mercenier A, Nauta A, Garcia-Rodenas CL. Wells JM, et al. Am J Physiol Gastrointest Liver Physiol. 2017 Mar 1;312(3):G171-G193. doi: 10.1152/ajpgi.00048.2015. Epub 2016 Dec 1. Am J Physiol Gastrointest Liver Physiol. 2017. PMID: 27908847 Free PMC article. Review.
-
Yang J, Qin K, Wang Q, Yang X. Yang J, et al. Int J Biol Macromol. 2024 Apr;264(Pt 2):130677. doi: 10.1016/j.ijbiomac.2024.130677. Epub 2024 Mar 6. Int J Biol Macromol. 2024. PMID: 38458298 Review.
Cited by
-
Brandi G, Calabrese C, Tavolari S, Bridonneau C, Raibaud P, Liguori G, Thomas M, Di Battista M, Gaboriau-Routhiau V, Langella P. Brandi G, et al. Int J Mol Sci. 2024 Jun 4;25(11):6182. doi: 10.3390/ijms25116182. Int J Mol Sci. 2024. PMID: 38892368 Free PMC article.